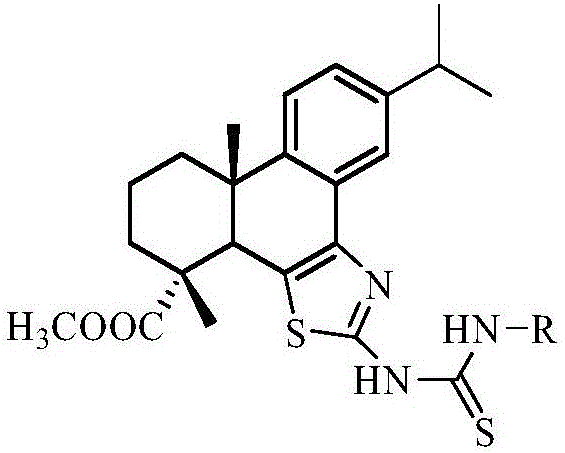
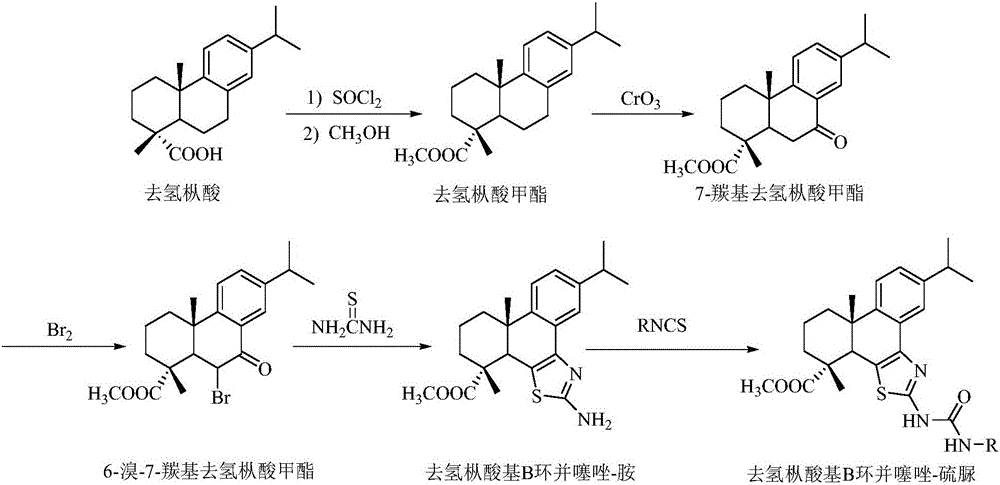
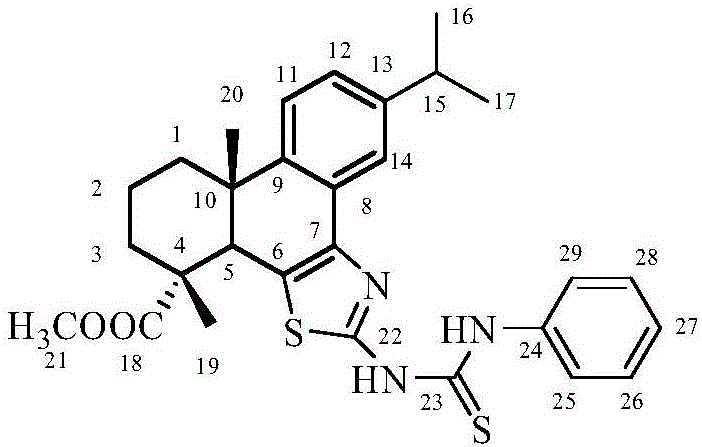
Synthesis method of dehydroabietic-acid-based B ring-fused-thiazole-thiocarbamide compounds
A technology of dehydroabietic acid and compounds, applied in the direction of biocides, organic chemistry, chemicals for biological control, etc., can solve the problems that have not been reported at home and abroad, and achieve the effect of increasing added value
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0025] The preparation of compound a:
[0026]
[0027] Into a 25mL two-necked flask, add 0.20g of dehydroabietyl B cyclothiazol-amine, 0.10g of phenylisothiocyanate and 5mL of acetonitrile, heat to reflux under stirring, react for 8h, and depressurize after the reaction is completed Acetonitrile was distilled off, and the residue was separated and purified by silica gel column chromatography. The eluent was ethyl acetate:petroleum ether=1:10 by volume to obtain compound a. m.p.175.0~176.0℃; IR(KBr,ν / cm -1 ):3149.22(ν N-H ), 2955.76, 2853.49 (ν Cα-H ),1726.96(ν C=O ),1599.71(ν C=S ), 1511.11, 1459.49 (ν Ar-C=C ),1382.53(ν C-N ),1029.97(ν C-O-C ); 1 H NMR (600MHz, CDCl 3 )δ: 13.31 (s, 1H, Ph-NH), 9.98 (s, 1H, thizole-NH), 7.89 (s, 2H, H-Ph), 7.63 (d, J=1.5Hz, 1H, H-14 ),7.47(t,J=7.9Hz,2H,H-Ph),7.30(d,J=7.4Hz,1H,H-Ph),7.23(d,J=8.0Hz,1H,H-11), 7.19 (dd, J=8.0, 1.8Hz, 1H, H-12), 3.78 (s, 1H, H-5), 3.69 (s, 3H, COOCH 3 ),2.94(m,1H,H-15),2.34(d,J=9.3Hz,1H,H 1-e ),1.9...
Embodiment 2
[0029] Preparation of compound b
[0030]
[0031] Into a 25mL two-necked flask, add 0.20g of dehydroabietyl B cyclothiazol-amine, 0.11g of m-fluorophenyl isothiocyanate and 5mL of acetonitrile, heat to reflux under stirring, and react for 8 hours. Acetonitrile was distilled off under reduced pressure, and the residue was separated and purified by silica gel column chromatography. The eluent was ethyl acetate:petroleum ether=1:10 by volume to obtain compound b. m.p.187.0~188.0℃; IR(KBr,ν / cm -1 ):3155.81(ν N-H ),2954.44(ν Cα-H ),1727.40(ν C=O ),1612.42(ν C=S ), 1508.74, 1450.45 (ν Ar-C=C ),1382.07(ν C-N ),1031.71(ν C-O-C ); 1 H NMR (600MHz, CDCl 3 )δ:13.53(s,1H,Ph-NH),10.48(s,1H,thizole-NH),7.95(s,1H,H-Ph),7.63(s,1H,H-14),7.58(s ,1H,H-Ph),7.40(dd,J=14.8,7.7Hz,1H,H-Ph),7.24(d,J=8.0Hz,1H,H-11),7.21(dd,J=8.0, 1.6Hz, 1H, H-12), 6.98(t, J=7.4Hz, 1H, H-Ph), 3.80(s, 1H, H-5), 3.73(s, 3H, COOCH 3 ),2.95(m,1H,H-15),2.35(d,J=6.0Hz,1H,H 1-e),1.89(m,5H,H-3,H-2,H 1-a ),1.63...
Embodiment 3
[0033] Preparation of compound c:
[0034]
[0035] Into a 25mL two-necked flask, add 0.20g of dehydroabietyl B cyclothiazol-amine, 0.11g of p-fluorophenylisothiocyanate and 5mL of acetonitrile, heat to reflux under stirring, and react for 8h. After the reaction is completed, Acetonitrile was distilled off under reduced pressure, and the residue was separated and purified by silica gel column chromatography. The eluent was ethyl acetate:petroleum ether=1:10 by volume to obtain compound c. m.p.191.0~192.0℃; IR(KBr,ν / cm -1 ):3280.93(ν N-H ),2951.97(ν Cα-H ),1713.10(ν C=O ),1623.29(ν C=S ), 1506.74, 1434.68 (ν Ar-C=C ),1378.11(ν C-N ),1035.27(ν C-O-C )cm -1 ; 1 H NMR (600MHz, CDCl 3 )δ: 13.22 (s, 1H, Ph-NH), 10.01 (s, 1H, thizole-NH), 7.80 (s, 2H, H-Ph), 7.58 (d, J=1.6Hz, 1H, H-14 ),7.23(d,J=8.0Hz,1H,H-11),7.20(dd,J=8.0,1.8Hz,1H,H-12),7.16(t,J=8.6Hz,2H,H-Ph ),3.79(s,1H,H-5),3.69(s,3H,COOCH 3 ),2.94(dq,J=13.8,6.9Hz,1H,H-15),2.34(d,J=5.9Hz,1H,H 1-e ),1.94–1.80(m,5H...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com