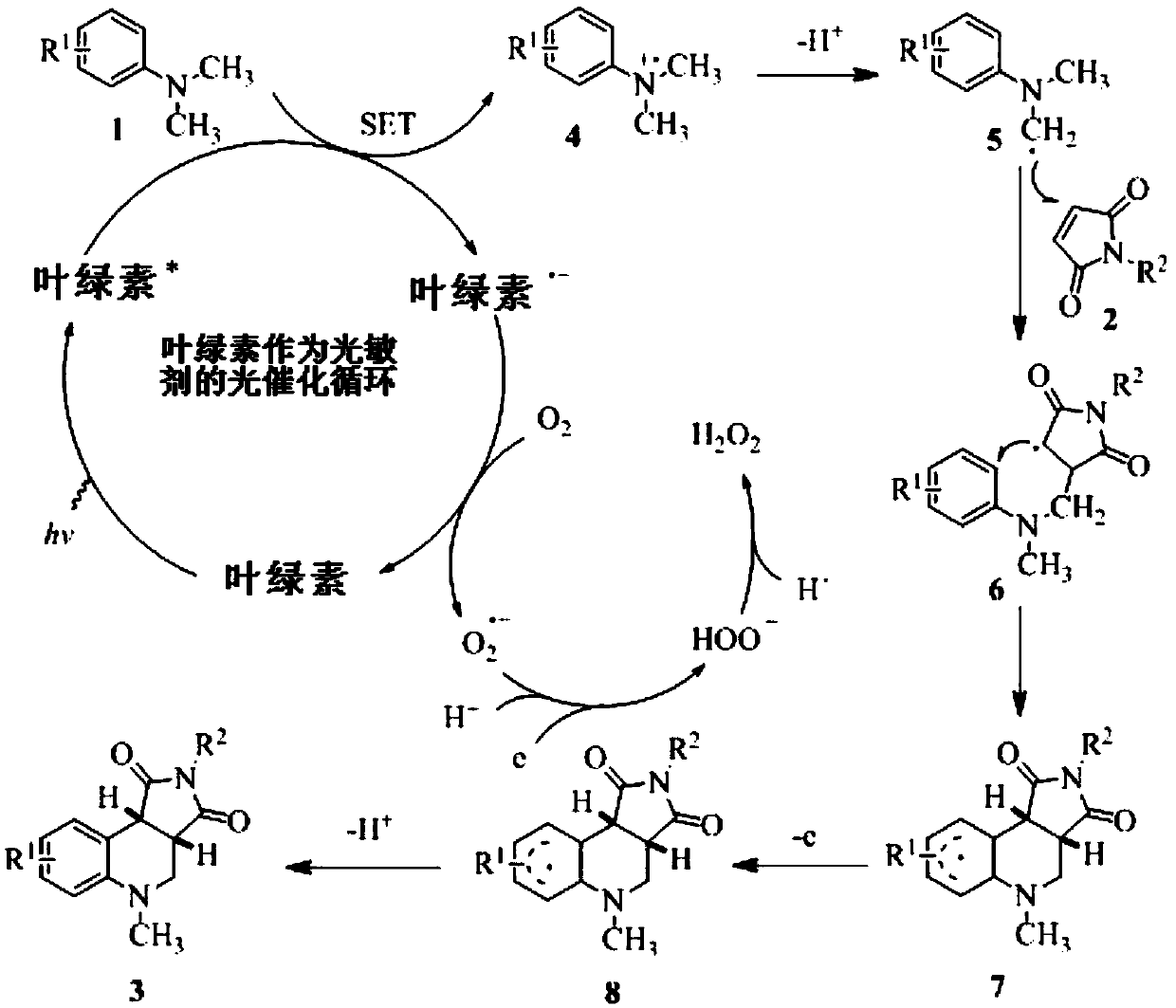
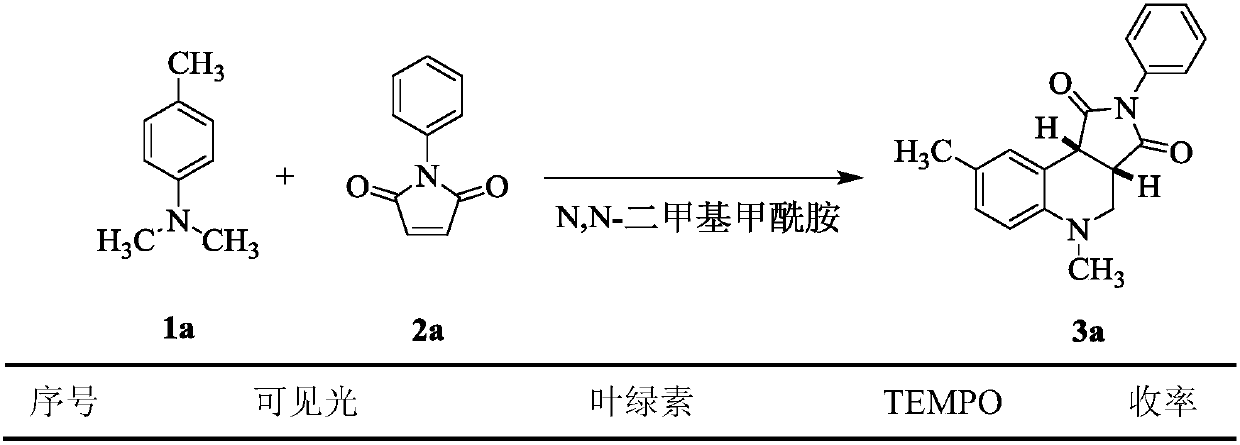
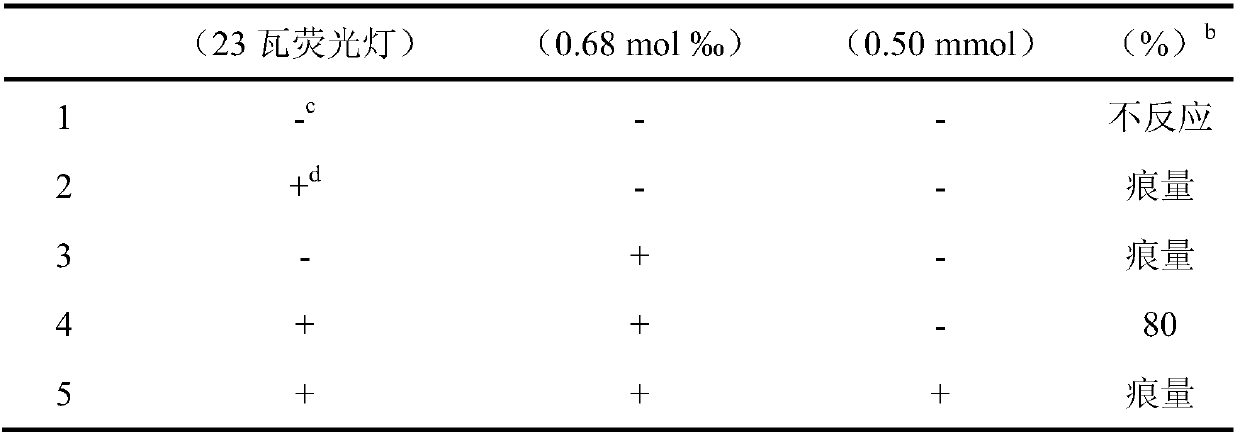
Application of chlorophyll as a photosensitizer in the synthesis of tetrahydroquinoline derivatives by visible light-catalyzed cyclization reaction
A cyclization reaction, tetrahydroquinoline technology, applied in the direction of organic chemistry, etc., can solve the problems of limited wide application, high price of rare metals, etc., and achieve the effect of high yield and green synthesis route
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0020] Preferred embodiments of the present invention are described in detail below. For the experimental methods that do not specify specific conditions in the examples, usually follow the conventional conditions or the conditions suggested by the manufacturer.
[0021] Main instruments and reagents used in this implementation method:
[0022] Main instruments: nuclear magnetic resonance instrument: model Bruker AVANCE DMX600, solvents are deuterated chloroform and deuterated dimethyl sulfoxide, tetramethylsilane is used as internal standard; rotary thin film evaporator.
[0023] Main reagents: chlorophyll (produced by Tokyo Chemical Industry Co., Ltd., Japan, extracted from plants, added dry gum arabic and lactose, and the content of chlorophyll in plant sources is 0.5%), N,N-dimethylaniline, N,N-di Methyl-p-toluidine, N,N-dimethyl-p-fluoroaniline, N,N-dimethyl-p-chloroaniline, N,N-dimethyl-p-bromoaniline, maleimide, N-methylmaleimide Laimide, N-ethylmaleimide, N-tert-buty...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com