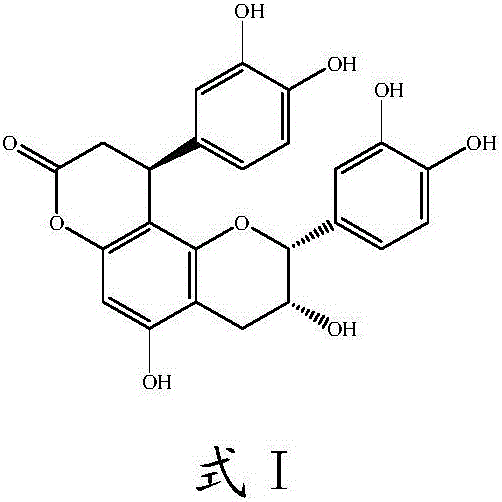
Preparation method and application of cinchonain Ib
A technology containing cinerene and plants, applied in the direction of effective components of heterocyclic compounds, metabolic diseases, cardiovascular system diseases, etc., can solve the problem of high-efficiency extraction of Ib without cinerene, achieve high safety, lower blood lipids, good synergistic effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
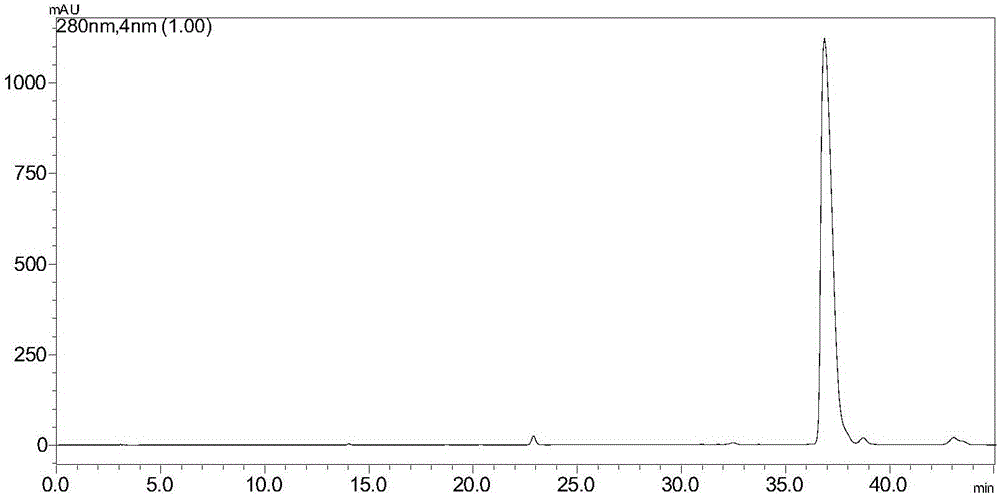
[0026] Example 1 The liquid phase detection method of cinchonine Ib
[0027] Chromatographic column: C-18 250×4.6mm
[0028] Detection wavelength: 280nm
[0029] Flow rate: 1.0ml / min
[0030] Mobile phase: A: 1% phosphoric acid
[0031] B: acetonitrile: water = 40:60
[0032] Detecting the liquid chromatographic peaks of cinchonaine Ib such as figure 1 shown.
Embodiment 2
[0033] Example 2 Extraction of cinchonaine Ib from apple polyphenols
[0034] 1) Add 2000 mL of water to 200 g of apple polyphenols (provided by Tianjin Jianfeng Natural Products Research and Development Co., Ltd., with a polyphenol content of 80%) and stir to dissolve at room temperature, and set aside;
[0035] 2) Macroporous resin adsorption separation: the apple polyphenol solution in 1) is adsorbed by HP20 type macroporous resin, and water, volume percentage concentration is 10%, 30%, 50%, 70%, 95% ethanol aqueous solution gradient elution , each half of the column bed volume is used as a receiving volume, and the fractions rich in cinchonaine Ib are collected by thin-layer chromatography combined with analytical HPLC detection, and combined, at a temperature ≤ 60 ° C, a vacuum of Concentrated under reduced pressure at 0.06-0.08 MPa, with a sugar content of 40, to obtain the primary enrichment of Xinconine Ib;
[0036] 3) Silica gel column separation: the primary enric...
Embodiment 3
[0042] Example 3 Extraction of cinchonaine Ib from grape vines
[0043] 1) 20 kg of grape vines were continuously extracted twice with 8-fold and 6-fold amounts of 60% ethanol, combined the extracts, concentrated under reduced pressure to a sugar content of 40, added 2 times the amount of water to disperse, and set aside.
[0044] 2) Macroporous resin adsorption separation: the grapevine extract solution obtained in 1) is adsorbed by HP20 type macroporous resin, water, volume percentage concentration is 10%, 30%, 50%, 70%, 95% ethanol aqueous solution gradient For elution, each half of the column bed volume is used as a receiving volume, and the components rich in cinchonaine Ib are collected by thin-layer chromatography combined with analytical HPLC detection, combined, at a temperature ≤ 60 ° C, vacuum Concentrated under reduced pressure with a concentration of 0.06-0.08MPa, a sugar content of 40, and a primary enrichment of Xinconine Ib with a content of 8.2%;
[0045] 3...
PUM
| Property | Measurement | Unit |
|---|---|---|
| wavelength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com