A pyrrolophenothiazine-1,3-dione derivative and its preparation method and application
A technology of phenothiazine and derivatives is applied in the field of pyrrolophenothiazine-1,3-dione derivatives and their preparation, which can solve problems such as affecting the polymerization process of tau protein, and achieve protection of nerve cells and treatment of Alzheimer's disease. Zheimer's disease, the effect of strong stabilizing effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
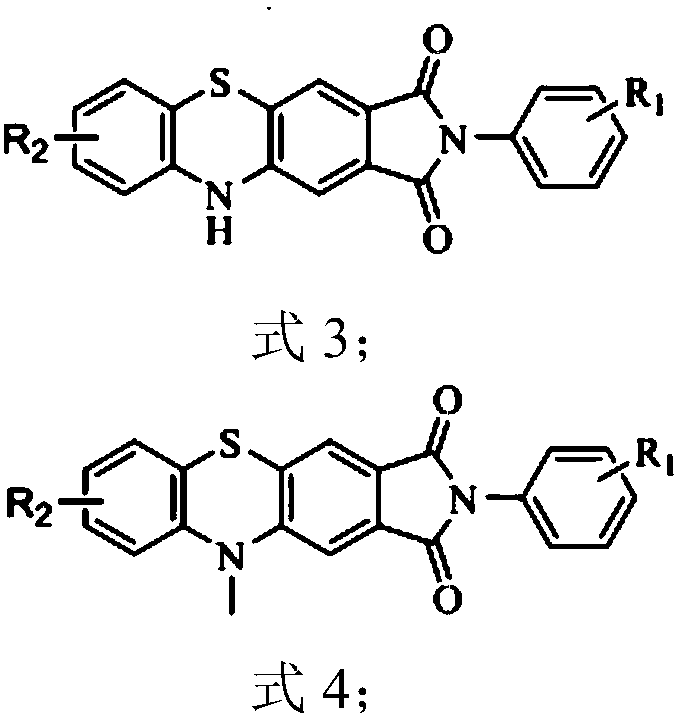
[0027] Synthesis of 2-(2,6-diisopropylphenyl)pyrrolo[3,4-b]phenothiazine-1,3(2H,10H)-dione 3a1
[0028] From the commercial compound 4,5-dichlorophthalic anhydride (430mg, 2mmol, 1eq) mixed with 2,6-diisopropylaniline (387mg, 2.2mmol, 1.1eq) in glacial acetic acid (10mL) Finally, the reaction temperature was raised to 120°C and refluxed for nearly 8 hours. The color change of the reaction solution could be observed, and the degree of reaction was detected by TLC. After the reaction solution was spin-dried, it was purified by column chromatography to obtain compound 1a, melting point : 180°C, yield 98%.
[0029] 1 H NMR (400MHz, CDCl 3 )δ8.06(s, 2H), 7.47(t, J=7.8Hz, 1H), 7.28(d, J=7.8Hz, 1H), 2.64(hept, J=7.8Hz, 2H), 1.16(d, 12H).
[0030] Afterwards, compound 1a (400mg, 1.06mmol, 1eq) was added into N,N-dimethylformamide (6mL) in which 2-aminothiophenol (133mg, 1.06mmol, 1eq) was dissolved, and then slowly added dropwise Triethylamine (737μL, 5.3mmol, 5eq), after uniform...
Embodiment 2
[0035] Synthesis of 7-chloro-2-(2,6-diisopropylphenyl)pyrrolo[3,4-b]phenothiazine-1,3(2H,10H)-dione 3a2 from commercially available 2- Amino-5-chlorothiophenol (424mg, 2.66mmol, 1eq) and compound 1a (1g, 2.66mmol, 1eq) refer to the synthesis process of Example 1 to obtain compound 3a2, a red solid, melting point: melting point: >300 ° C, The total yield is 54%.
[0036] 2a2: Melting point: 259℃, 1 H NMR (400MHz, CDCl 3 )δ:7.94(s,1H),7.45(m,2H),7.33(dd,J=7.5,3.0Hz,1H),7.28(d,J=7.5Hz,2H),7.17(s,1H), 6.84 (d, J=7.5Hz, 1H), 2.67 (hept, J=7.0Hz, 2H), 1.16 (d, J=7.5Hz, 12H).
[0037] 3a2: 1 H NMR (400MHz, DMSO) δ9.55(s, 1H), 7.51(s, 1H), 7.46(t, J=7.5Hz, 1H), 7.31(d, J=7.5Hz, 1H), 7.10-7.06 (m,2H),7.01(s,1H),6.67(d,J=9.0Hz,1H),2.61(hept,J=7.0Hz,2H),1.06(dd,J=7.0,2.0Hz,12H) ;ESI-MS m / z:463.2[M+H] + .
Embodiment 3
[0039] Synthesis of 8-chloro-2-(2,6-diisopropylphenyl)pyrrolo[3,4-b]phenothiazine-1,3(2H,10H)-dione 3a3 from commercial 2- Amino-4-chlorothiophenol (382mg, 2.39mmol, 1eq) and compound 1a (900mg, 2.39mmol, 1eq) refer to the synthesis process of Example 1 to obtain compound 3a3, a red solid, melting point: 340 ° C, total yield 55%.
[0040] 2a3: Melting point: 267℃, 1 H NMR (400MHz, CDCl 3 )δ:7.42-7.46(m,2H),7.29(s,1H),7.1(s,1H),6.84(m,2H),6.6(s,1H),6.44(d,J=3.0Hz,1H ), 2.67 (hept, J=7.0Hz, 2H), 1.16 (d, J=7.0Hz, 12H).
[0041] 3a3: 1 H NMR (400MHz, CDCl 3 )δ7.42-7.46(m,2H),7.29(d,J=7.5Hz,2H),7.10(s,1H),6.84(m,2H),6.54(s,1H),6.45(d,J =3.0Hz, 1H), 2.68(hept, J=7.0Hz, 2H) 1.16(d, J=7.0Hz, 12H); ESI-MS m / z: 463.2[M+H] + .
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com