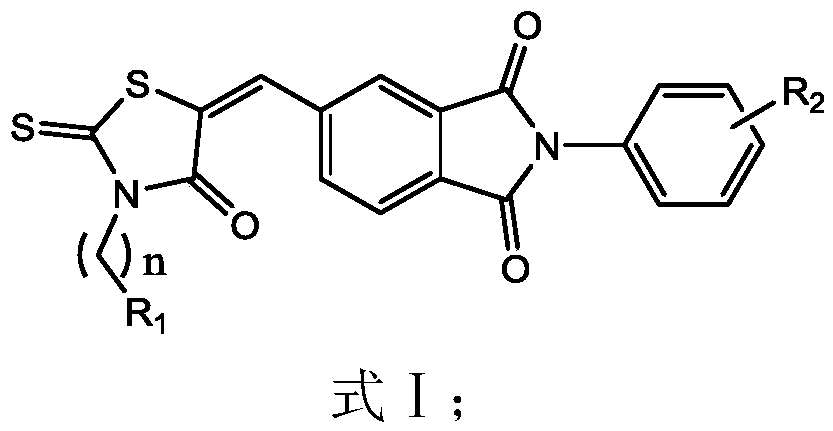
A kind of 4-oxo-2-thiothiazolidinyl derivative and its preparation method and application
A thiothiazolidine and derivative technology, applied in the field of biomedicine, can solve the problems of reduced activity of promoting microtubule assembly, impact on dynamic behavior of microtubules, cell death, etc., to prevent tubulin depolymerization and treat Alzheimer's disease. Hymer's disease, the effect of protecting nerve cells
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
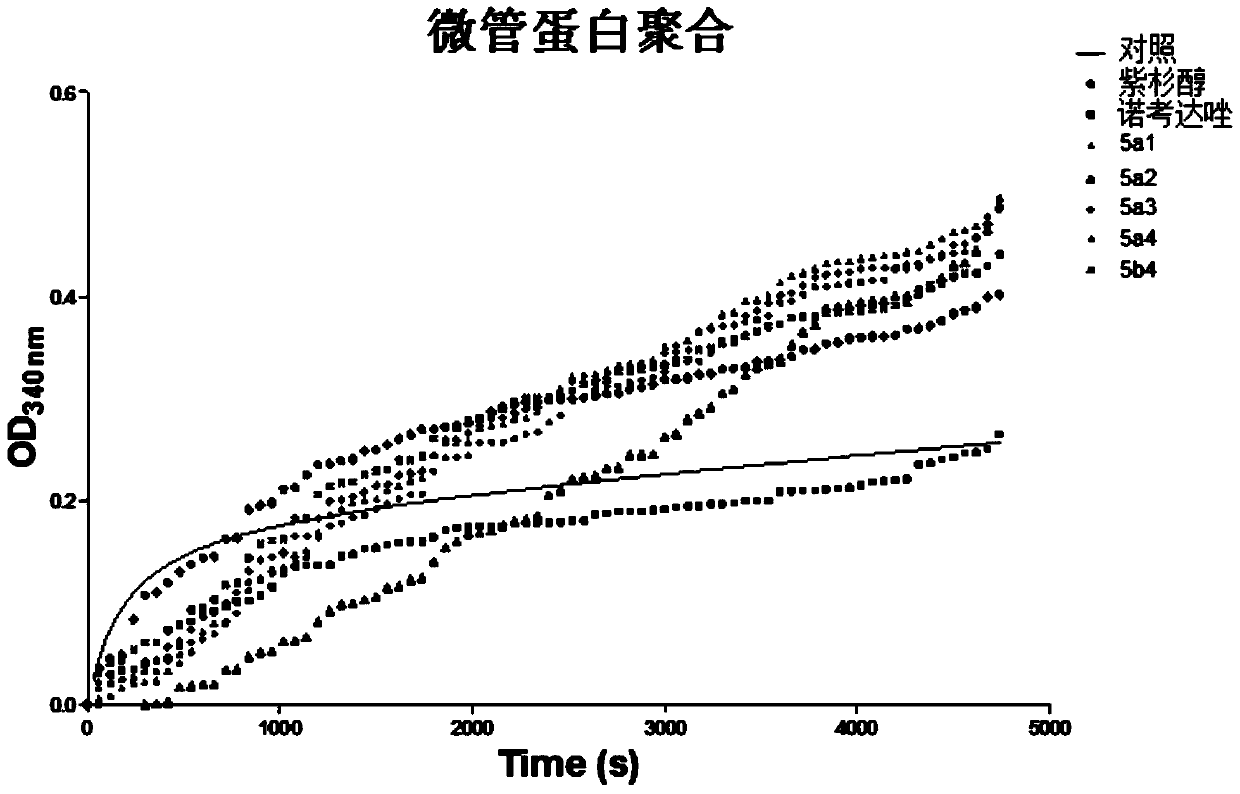
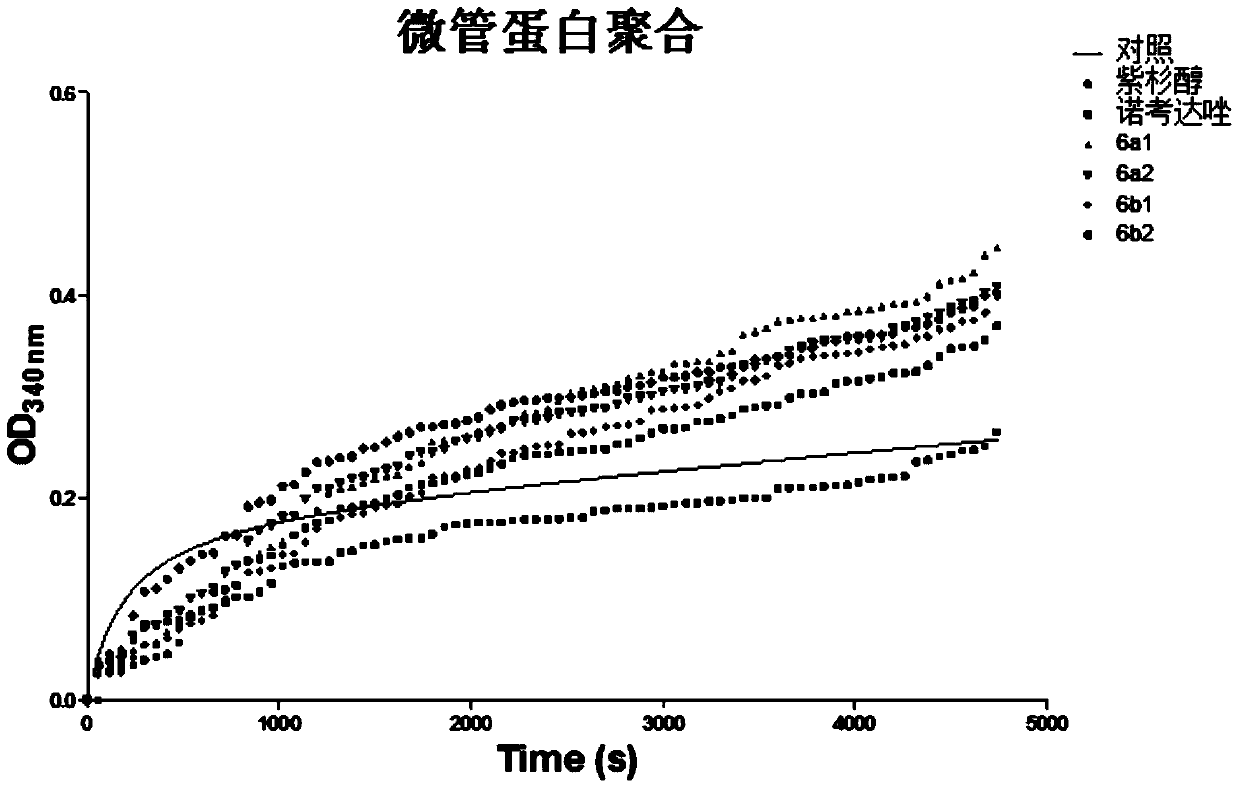
Image
Examples
Embodiment 1
[0028] Synthesis of ethyl 2-(4-oxo-2-thiothiazolidin-3-yl)acetate 1a1
[0029] Add glycine ethyl ester hydrochloride (500mg, 3.6mmol, 1eq) to 2,2'-(thiocarbonyl(thio))diacetic acid (976mg, 4.32mmol, 1.2eq) in isopropanol (4mL) , and then drop Et into the system 3 N (1 mL, 7.2 mmol, 2 eq). The reaction system was heated to 82°C and stirred for 1 h. After the reaction was monitored by TLC, after concentration to dryness, the product 1a1 (638mg, 2.91mmol) was obtained by silica gel column separation, a yellow crystalline solid, melting point: 55°C, yield 81%.
[0030] 1 H NMR (400MHz, CDCl 3 )δ=4.65(s, 2H), 4.19(q, J=7.8Hz, 2H), 4.01(s, 2H), 1.22(t, J=7.8Hz, 3H); ESI-MS m / z: 220.2[ M+H] + .
Embodiment 2
[0032]Synthesis of tert-butyl 2-(4-oxo-2-thiothiazolidin-3-yl)acetate 1a2
[0033] Glycine tert-butyl ester hydrochloride (500mg, 3mmol, 1eq) and trithiocarbonate (813mg, 3.6mmol, 1.2eq) were isolated according to the operation process of Example 1 to obtain compound 1a2 (504mg), yellow crystalline solid, Melting point: 56°C, yield 68%.
[0034] 1 H NMR (400MHz, CDCl 3 )δ=4.62(s,2H),4.07(s,2H),1.46(s,9H); ESI-MS m / z:248.2[M+H] + .
Embodiment 3
[0036] Synthesis of ethyl 2-(4-oxo-2-thiothiazolidin-3-yl)propionate 1b1
[0037] 3-aminopropionic acid ethyl ester hydrochloride (500mg, 3.27mmol, 1eq) and 2,2'-(thiocarbonyl (sulfur)) diacetic acid (886mg, 3.92mmol, 1.2eq), refer to the operation process of Example 1 , isolated product 1b1 (708mg), yellow crystalline solid, melting point: 53°C, yield 93%.
[0038] 1 H NMR (400MHz, CDCl 3 )δ4.30(t, J=7.8Hz, 2H), 4.18(q, J=7.8Hz, 2H), 4.00(s, 2H), 2.68(t, J=7.8Hz, 2H), 1.27(t, J=7.7Hz,3H); ESI-MS m / z:234.1[M+H] + .
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com