Application of n-formyl-3,4-methylenedioxybenzyl-γ-butyrolactone in the preparation of anti-hepatitis B virus medicine
A hepatitis B virus and drug technology, applied in the field of medicine, can solve the problem that there is no report on the inhibitory effect of hepatitis B virus
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
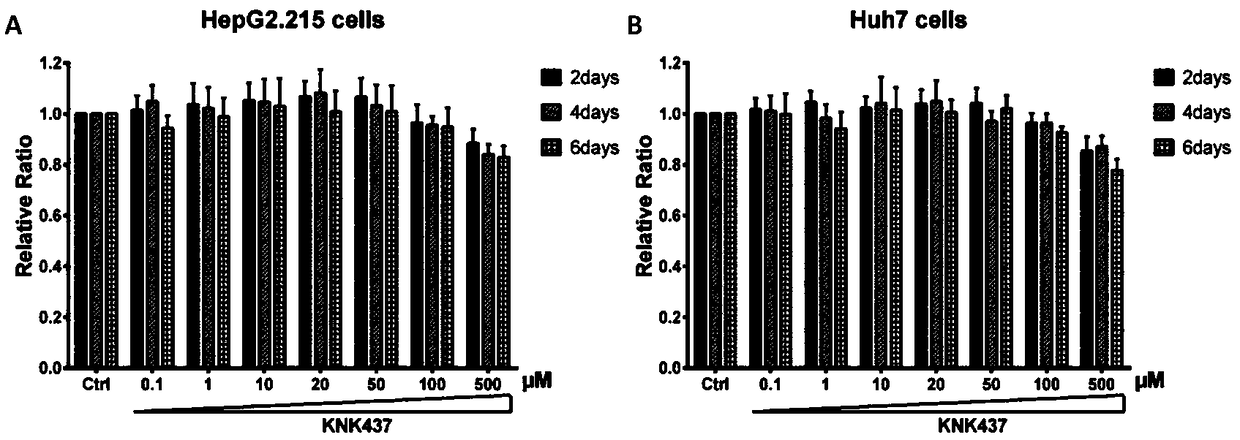
[0029] [Example 1] Cytotoxicity of KNK437 on HepG2.215 and Huh7 cell lines
[0030] HepG2.2.15 and HuH7 cells were treated with 10 4 The amount of cells / well was seeded in a 96-well plate, and after 12 hours of culture, different concentrations of KNK437 were added for stimulation. The concentrations of KNK437 were: 0 μM, 0.1 μM, 1 μM, 10 μM, 20 μM, 50 μM, 100 μM, 500 μM; treatment time 2 days, 4 days, and 6 days, respectively. In the final test, remove the supernatant, wash three times with PBS, and then add fresh serum-free medium containing 10% CCK-8 solution, and continue to incubate for 2 hours. Microplate reader to measure OD of each well 490 absorbance value. The absorbance value of the unmedicated control was taken as the relative value 1 of the cell growth rate, and the absorbance values of other wells were compared with it to obtain the relative value of the respective cell growth rate.
[0031] Experimental results ( figure 1 ), it can be seen that KNK437 has ...
Embodiment 2
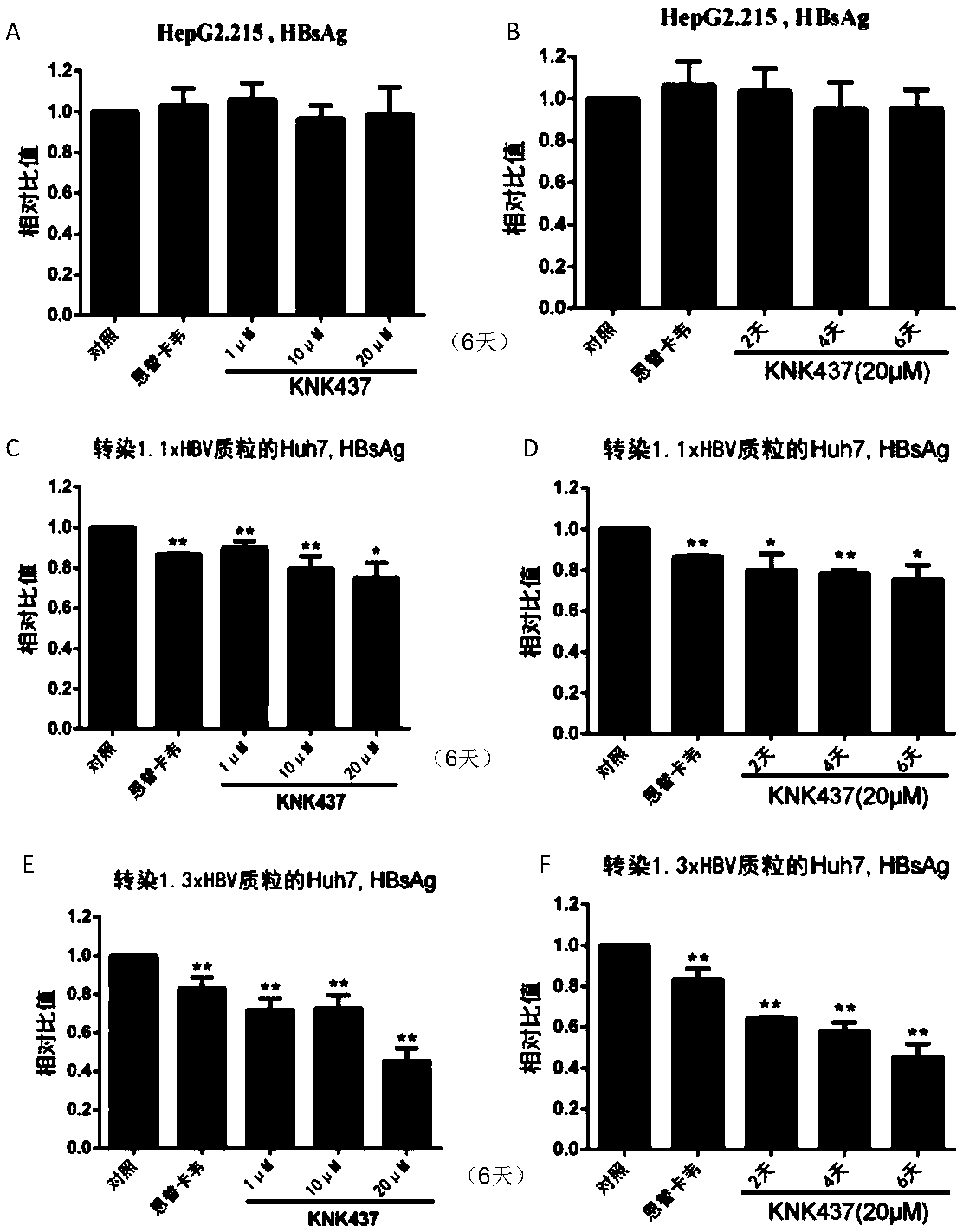
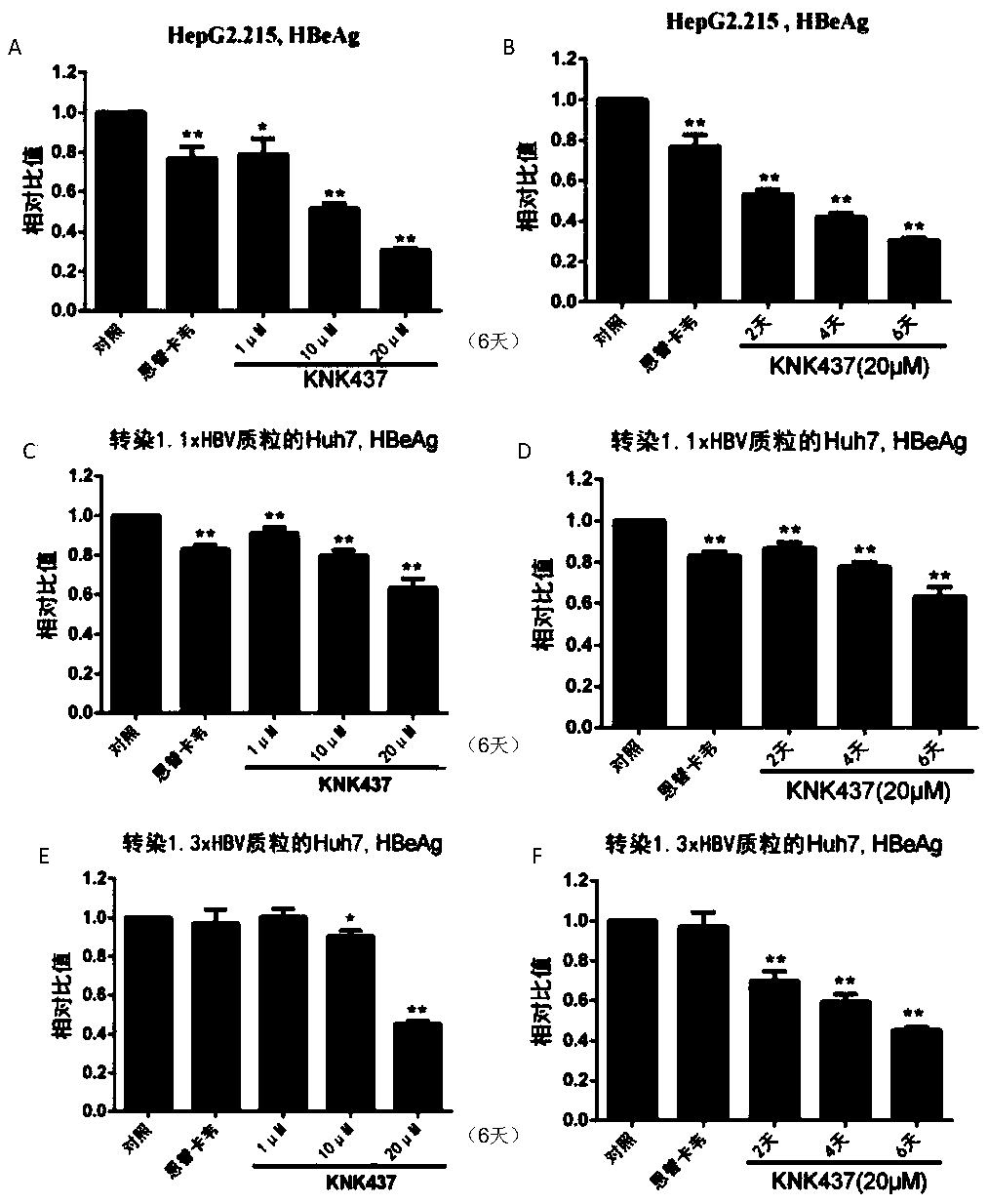
[0033] [Example 2] Detection of HBsAg and HBeAg in the supernatant of three liver cancer cell lines treated with KNK437
[0034] (1) Add 1 μM, 10 μM and 20 μM KNK437 to HepG2.2.15 and Huh7 cells transfected with 1.1 / 1.3×HBV plasmid, and 30 nM entecavir (ETV) as an anti-HBV positive control drug for 6 days, every two days The medium was changed, and the cell supernatant was stored at -40°C after 6 days of collection;
[0035] (2) 20 μM KNK437 was added to HepG2.2.15 and Huh7 cell culture medium transfected with 1.1 / 1.3×HBV plasmid, and the cells were treated for 2 days, 4 days, 6 days, and the positive control 30nM ETV for 6 days. Change the fresh culture medium every two days, and collect the cell supernatant at -40°C for use;
[0036] (3) The cell supernatants collected in (1) and (2) were thawed and centrifuged at 10,000 g for 5 minutes to remove cell residues, and then the supernatants were preheated in a 37°C incubator for 30 minutes;
[0037] (4) Take 500 μl of each sam...
Embodiment 3
[0046] [Example 3] Detection of viral mRNA levels in three liver cancer cell lines treated with KNK437
[0047] (1) 1μM, 10μM and 20μM KNK437, and 30nM entecavir (ETV) were added to HepG2.2.15 inoculated in six-well plate and Huh7 cell culture medium transfected with 1.1 / 1.3×HBV plasmid as anti-HBV positive control After 6 days of drug treatment, the medium was changed every two days. After 6 days, the corresponding RNAiso Plus lysis solution was added to each well of the cells. Generally, 1 ml of RNAiso Plus lysis solution was added to each well of a six-well cell culture dish. Place at room temperature for 5 minutes to fully lyse the cells, blow the cells evenly, and then collect them into a 1.5ml RNase-free centrifuge tube;
[0048] (2) 20 μM KNK437 was added to HepG2.2.15 inoculated in six-well plate and Huh7 cell culture medium transfected with 1.1 / 1.3xHBV plasmid, and the cells were treated for 2 days, 4 days, 6 days, and positive control 30nM ETV was treated for 6 days...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com