Process for preparing tetrabenzyl-voglibose hydrochloride
A tetrabenzyl voglibose and preparation technology, which is applied in the field of pharmaceutical synthesis, can solve the problems of complex waste liquid treatment, environmental pollution, explosion, etc., and achieve the effects of simplified process, less three wastes, and mild reaction temperature
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
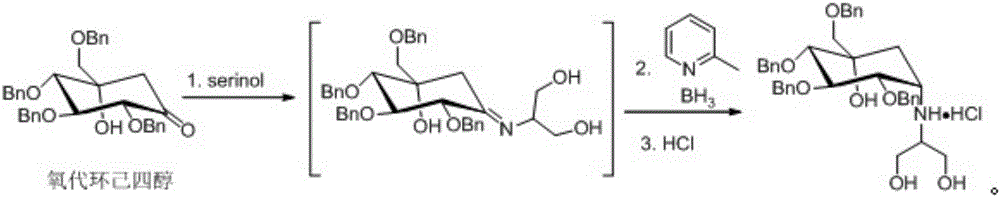
[0024] Add 100mL methanol, 2g serinol, 2ml formic acid to oxocyclohexyl tetraol (10g, 18mmol), stir for 1 hour at 15°C, add (1.93g, 18mmol) 2-picoline-N-methylboron alkanes at 15°C for 16 hours. The solvent was evaporated to dryness to obtain the crude product.
[0025] Add 120mL EA to the crude product, wash twice with 40mL water and twice with 40mL 0.5M HCl aqueous solution, stir the organic phase at 15°C for 16 hours, filter with suction and dry to obtain tetrabenzyl voglibose hydrochloride 8.7 g, yield 73%.
Embodiment 2
[0027] Add 100mL ethanol, 4g serinol, 2ml acetic acid to oxocyclohexyl tetraol (10g, 18mmol), stir for 1 hour at 20°C, add (1.93g, 18mmol) 2-picoline-N-methylboron alkanes at 20°C for 16 hours. The solvent was evaporated to dryness to obtain the crude product.
[0028] Add 100mLEA to the crude product, wash twice with 50mL water and twice with 50mL 1M HCl aqueous solution, stir the organic phase at 20°C for 16 hours, filter with suction and dry to obtain 7.3g of tetrabenzyl voglibose hydrochloride, Yield 61%.
Embodiment 3
[0030] Add 100mL ethanol, 3g serinol, 2ml acetic acid to oxocyclohexyl tetraol (10g, 18mmol), stir for 1 hour at 30°C, add (1.93g, 18mmol) 2-picoline-N-methylboron alkanes at 20°C for 16 hours. The solvent was evaporated to dryness to obtain the crude product.
[0031] Add 100mLEA to the crude product, wash twice with 50mL water and twice with 50mL 0.5M HCl aqueous solution, stir the organic phase at 20°C for 16 hours, filter with suction and dry to obtain 6.2g of tetrabenzyl voglibose hydrochloride , yield 53%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com