Tildipirosin hexamethylene compound and preparation method
A new technology of solvent compound and Tiderol, which is applied in the preparation of sugar derivatives, organic chemical methods, chemical instruments and methods, etc., can solve the problems of small particles, easy aggregation, poor fluidity, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0026] Preparation of Tedirosine Cyclohexane Solvent Compound
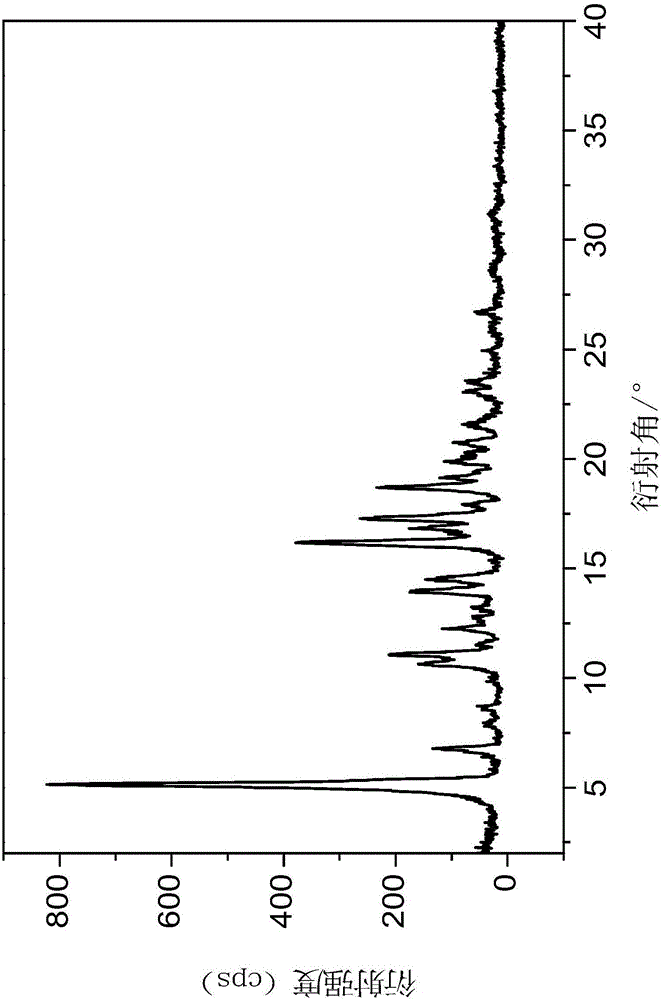
[0027] Keep the temperature constant at 20°C, add 30g of cyclohexane into the crystallizer, add 6g of Tidelotide raw material into the solution, after adding the raw material, the system will have a solid suspension, continue to stir for 2 hours, filter the product, and place it in a 70°C drying oven Dry under normal pressure for 4 hours to obtain the final product. The powder diffraction pattern of the analysis product is as attached figure 1 As shown, the spectrum starts at 2θ=5.14 ° and the strongest peak; the specific data is shown in the following table:
[0028]
[0029]
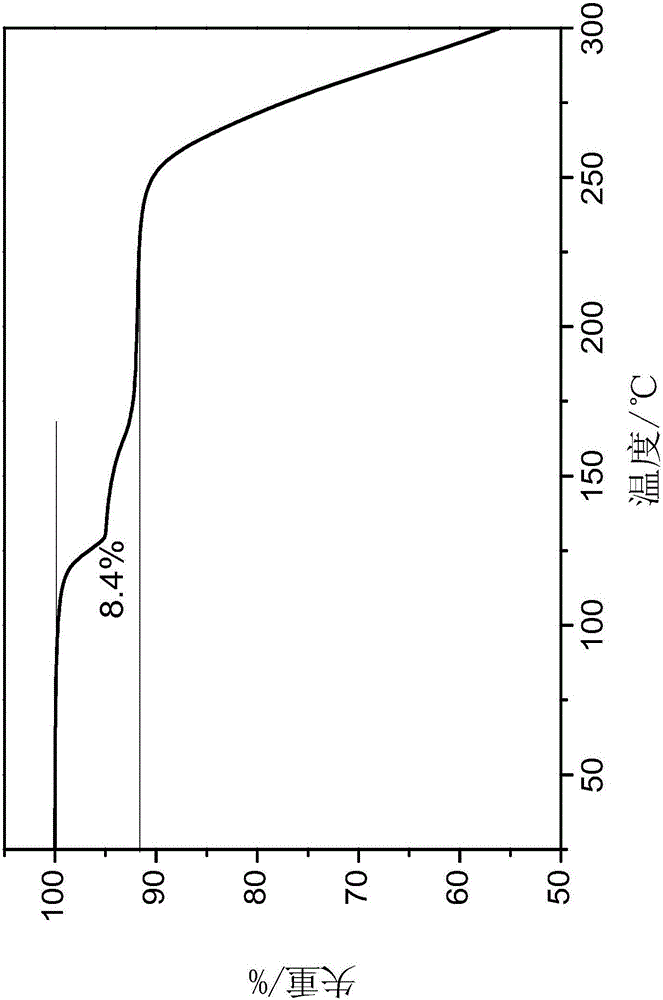
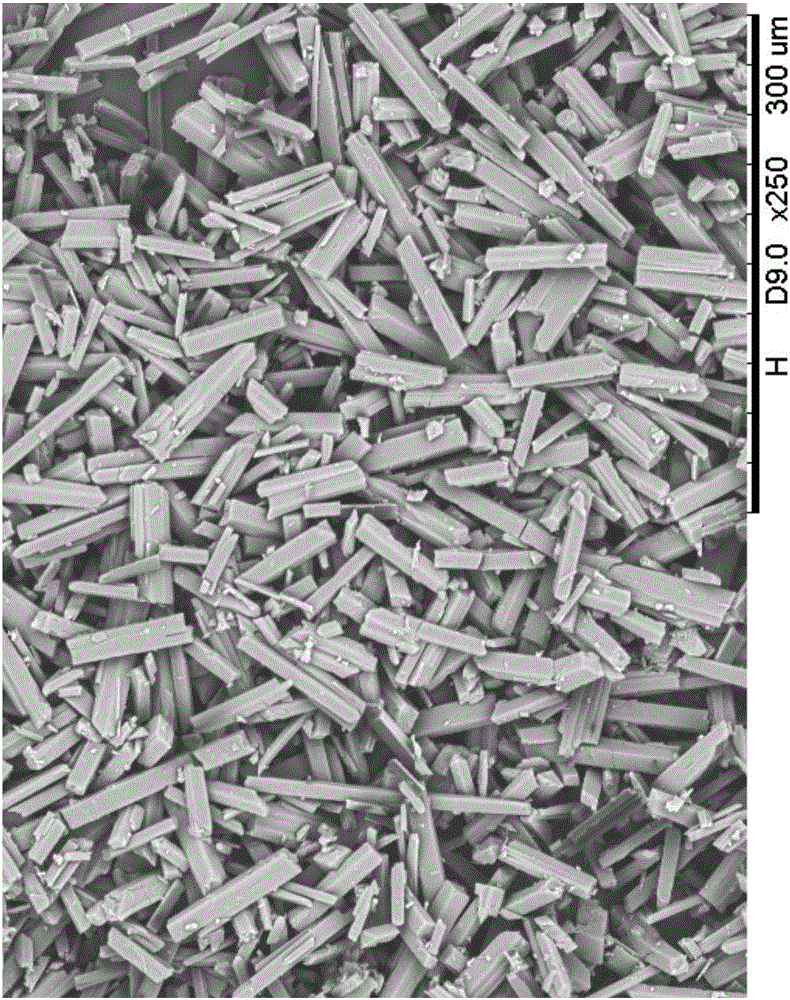
[0030] The results of thermogravimetric analysis of the samples are attached figure 2 As shown, the weight loss is 8.4%, indicating that what is obtained is the tedirosine cyclohexane solvent compound; the scanning electron microscope SEM photo of the product is as attached image 3 As shown, it is rod-shaped.
Embodiment 2
[0032] Preparation of Tedirosine Cyclohexane Solvent Compound
[0033] Keep the temperature at 60°C, add 45g of cyclohexane and 15g of Tidelotol new raw materials into the crystallizer at one time, stir to make the system in a uniform suspension state, filter the white solid after 0.5h, filter the product, and place it in a 60°C drying oven under normal pressure Dry for 4h to get the final product. Analyze the powder diffraction pattern of the product and the attached figure 1 The spectrum has the same peak spectrum position and shape, and the spectrum is at 2θ=5.1 ° initial peak and the strongest peak; thermogravimetric analysis is as attached Figure 4 Shown, weight loss is 10.8%, what illustrate to obtain is tedirosine cyclohexane solvent compound; Product is observed under microscope and attached image 3 Similar, rod-shaped.
Embodiment 3
[0035] Preparation of Tedirosine Cyclohexane Solvent Compound
[0036] Keep the temperature at 30°C, add 40g of cyclohexane and 4g of Tidelotol new raw materials into the crystallizer at one time, stir to make the system in a uniform suspension state, filter the white solid after 4h, filter the product, and place it in a 65°C drying oven to dry under normal pressure 5h to obtain the final product. Analyze the powder diffraction pattern of the product and the attached figure 1 The spectrum has the same peak spectrum position and shape, and the spectrum is at 2θ=5.1 °. The initial peak and the strongest peak; the thermogravimetric analysis results show that the weight loss is 9.4%, indicating that what is obtained is the tedirosine cyclohexane solvent compound; the product is under the microscope Observation and Attachment image 3 Similar, rod-shaped.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com