18F click labelling transferrin receptor targeting polypeptide T7 as well as preparation method and application thereof
A transferrin, targeting peptide technology, applied in the preparation methods of peptides, chemical instruments and methods, peptides, etc., can solve the problems of long time, unfavorable automatic synthesis, low radioactive recovery rate, etc., and achieve radiochemical yield. High, the effect of reducing one purification process and reducing the time of synthesis and purification
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 118
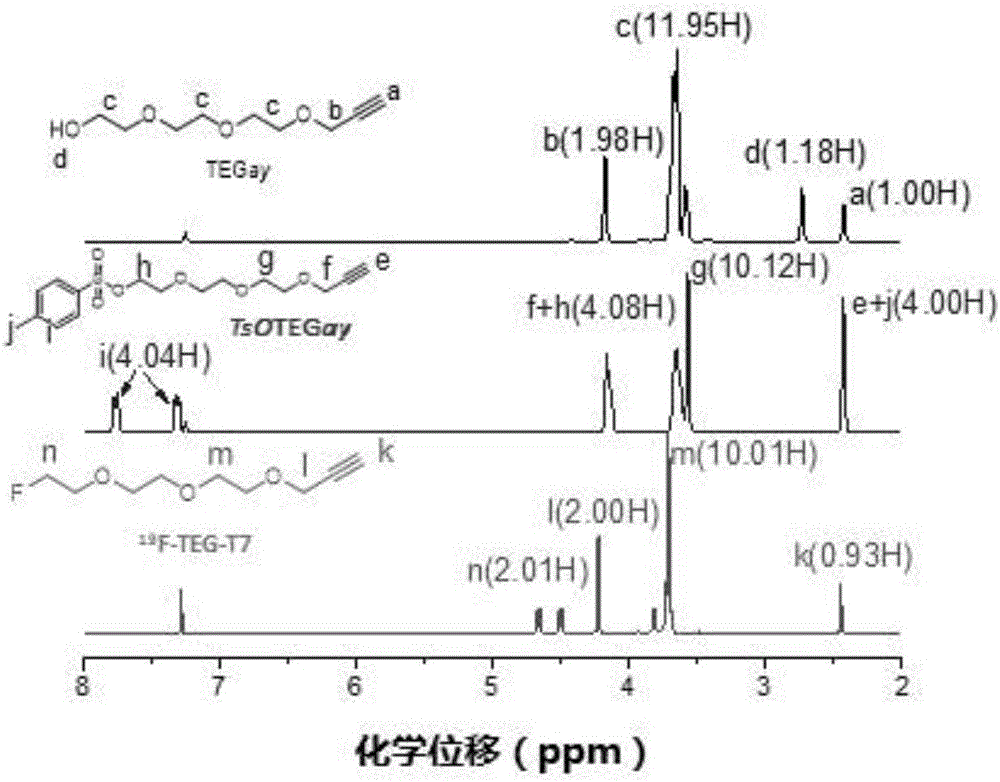
[0049] Example 1 18 Chemical Synthesis of F-TEG-T7
[0050] 1) Synthesis of labeled precursor TsOTEGay
[0051] At 0°C, slowly add 60% NaH (2g, 0.050mol) into a THF (60mL) solution of TEG (11.6g, 0.077mol) and stir. After no bubbles emerge, add 3-bromopropyne ( 2.1 mL, 0.039 mol), the reaction was followed by TLC (petroleum ether: ethyl acetate = 1:2). The solution changed from colorless to brown, and the reaction was completed. The reaction solution was centrifuged, the solvent was concentrated, and purified by column chromatography with petroleum ether: ethyl acetate = 1:2-1:4. The yellow liquid product (TEGay) was obtained in 3.58 g, the yield was 49%.
[0052] Dissolve TEGay (2.00 g, 0.010 mol) p-hydroxybenzoyl chloride (0.012 mol) in 50 mL of anhydrous CH 2 Cl 2 , cooled to 0°C. Add 2.98g (0.053mol) KOH solid in 10 times, and stir at 0°C for 1h. Stir at room temperature for another 5 h (TLC to track the reaction, petroleum ether: ethyl acetate = 2:1). After the re...
Embodiment 2
[0058] Example 2 Standards 19 Chemical Synthesis of F-TEG-T7
[0059] H-TEGay (100 mg, 0.5 mmol) was dissolved in 2.5 mL of anhydrous dichloromethane and cooled to 0 °C. DAST (132 μL, 1 mmol) was added slowly, and after reacting for 1 h at 0° C., the reaction was continued at room temperature for 5 h. After the reaction, the product was separated with a silica gel column (eluent: ethyl acetate:petroleum ether=1:6~1:3), and the light yellow oily product was obtained after drying the solvent.
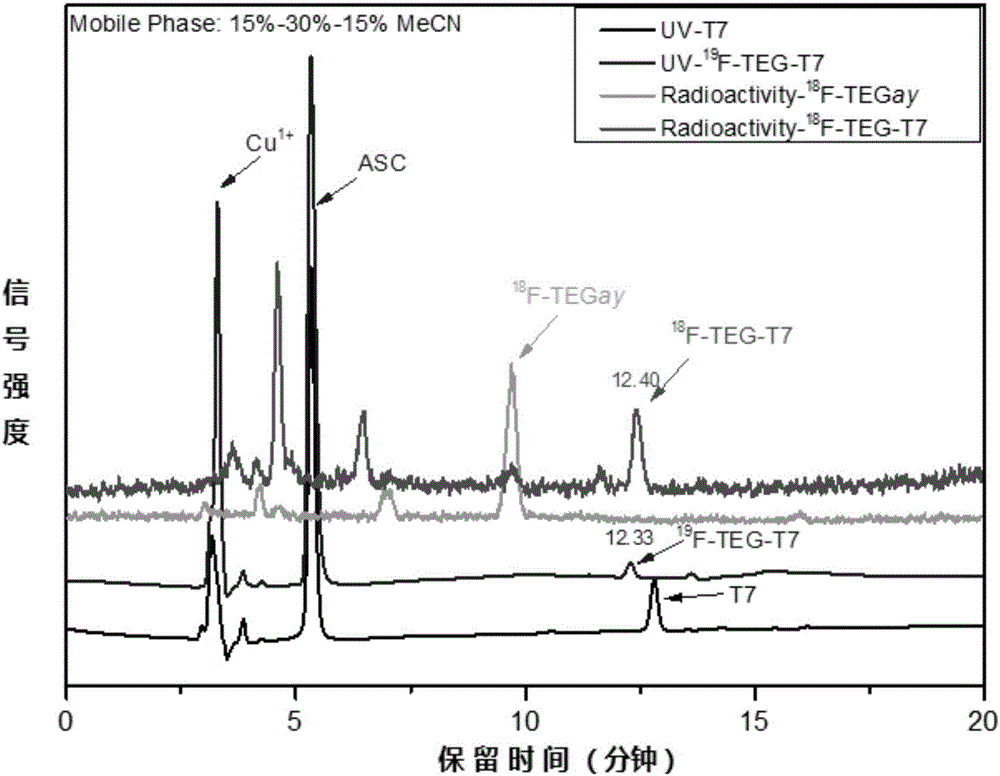
[0060] The collected product was characterized by NMR spectroscopy as 19 F-TEGay, for second-step click reaction synthesis of standards 19 F-TEG-T7. 19 F-TEGay (0.34mg, 1.79μmol) was dissolved in 400μL acetonitrile, T7 polypeptide (2mg, 1.79μmol), CuSO 4 .5H 2 O (2.2mg, 8.9μmol) and ASC (14.2mg, 71μmol) were dissolved in 800μL H 2 In O, mix acetonitrile and aqueous solution, seal, and react at 60°C for 30min. After the reaction, HPLC was used for detection and separation, and afte...
Embodiment 3
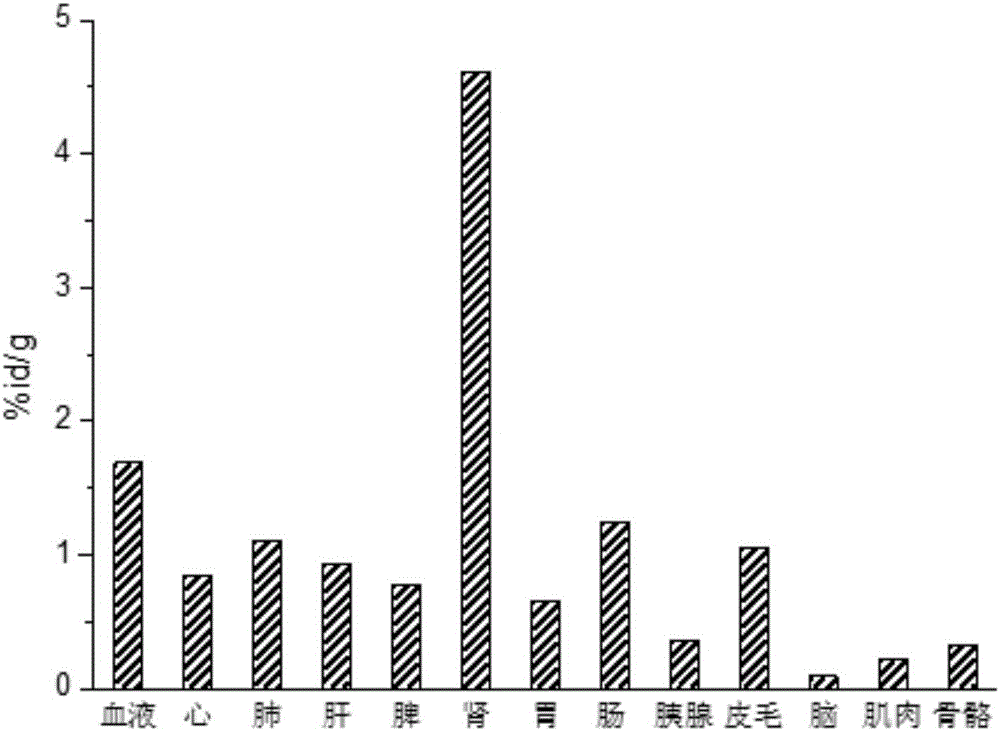
[0067] Example 3 18 Lipid-water partition coefficient experiment of F-TEG-T7
[0068] Take 100μL 18 The F-TEG-T7PBS preparation was placed in a 1.5mL centrifuge tube (containing 500μL n-butanol and 400μL PBS), sealed, vortexed at room temperature for 2min, and then centrifuged at high speed for 3min (10000r / min) until the two phases were balanced. Use a pipette gun to sample 100 μL each from the organic phase and the aqueous phase and place them in two gamma counter tubes to determine the gamma count. Repeat the sampling measurement 3 times. The average lg D is calculated according to the following formula 7.4 value.
[0069] d 7.4 =lg(N 1 / N 2 ); where: N 1 , radioactive count rate per ml n-octanol, min -1 ·mL -1 ; N 2 , radioactive count rate per ml buffer solution PBS, min -1 ·mL -1 .
[0070] Calculated by measurement, 18 The lipid-water partition coefficient of F-TEG-T7 is -0.51±0.01, and the targeting polypeptide tends to be more hydrophilic. It can be pre...
PUM
| Property | Measurement | Unit |
|---|---|---|
| wavelength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com