A sort of 18 f-labeled compounds and pod protease-targeted pet imaging probes
A technology for compounds and precursor compounds, applied in the fields of radiopharmaceuticals and nuclear medicine, can solve the problem that positron emission tomography has not been applied, and achieve the effects of reducing non-specific uptake, promoting application and simple operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0073] 18 The F-labeled compound has a precursor compound of the structure shown in the following formula 1, and its synthetic route is,
[0074]
[0075] Intermediate compound 1-2 was synthesized according to the method reported in the literature [Lin, J. et al. Chem Commun (Camb) 2017, 53, (48), 6476-6479.],
[0076]
[0077] Weigh compound 1-2 (38mg, 0.08mmol), compound 1-1 (50mg, 0.09mmol) and HBTU (62mg, 0.16mmol), add it to the reaction bottle, and dissolve it with ultra-dry DMF ultrasonically for 1min (model: KQ2200E, power : 100W), then add DIPEA (88μL, 0.53mmol), N 2 Reaction at room temperature under protection for 2h. After separation and purification by column chromatography (eluent: DCM:MeOH (volume ratio) = 35:1 to 15:1, the solvent was collected and rotary evaporated, and dried in vacuum for 2 hours to obtain compound 1-3 (60 mg, yield 72%) .
[0078] The obtained compound 1-3 was dissolved in 4 mL of DCM:MeCN:TFA=1:1:2 (volume ratio), the solution tur...
Embodiment 2
[0083] 18 The F-labeled compound has a precursor compound of the structure shown in the following formula 2, and its synthetic route is,
[0084]
[0085] Polypeptide Ac-HEHEHEAAN-OH, compound 2-1, was synthesized by solid-phase peptide synthesis, and the synthetic route was as follows:
[0086] By sequentially linking Fmoc-N-trityl-L-asparagine (298 mg, 0.5 mmol), N-fluorenylmethoxycarbonyl-L-alanine (156 mg, 0.5 mmol), N-fluorenylmethoxycarbonyl- L-alanine (156mg, 0.5mmol), Fmoc-L-glutamic acid-5-tert-butyl ester hydrate (213mg, 0.5mmol), Fmoc-trityl-L-histidine (310mg, 0.5 mmol), Fmoc-L-glutamic acid-5-tert-butyl hydrate (213mg, 0.5mmol), Fmoc-trityl-L-histidine (310mg, 0.5mmol), Fmoc-L-glutamine Acid-5-tert-butyl hydrate (213mg, 0.5mmol), Fmoc-trityl-L-histidine (310mg, 0.5mmol), acetic anhydride (1mL, 10mmol), de-resin, rotary evaporation solvent, Ether was precipitated, and compound 2-1 was obtained after vacuum drying (634 mg, yield 56%).
[0087]
[0088] Com...
Embodiment 3
[0094] Radiosynthesis:
[0095] probe 18 The radiosynthetic route of F-1 compound is as follows,
[0096]
[0097] Produced on demand by a medical cyclotron 18 F fluoride ion. After production is complete, the 18 The F fluoride ion is passed to the anion exchange column (QMA), and the pyridazine buffer solution (300 μL, pH=2.5) is used to 18 F anion (150-300mCi) is eluted into polypropylene reaction tube (1mL) from QMA column, the AmBF synthesized in embodiment 1 3 -CBT-Cys(StBu)-AAN (precursor compound of the structure shown in formula 1, that is, probe precursor 1) (25mM, 30μL) was added to the reaction tube and incubated at 80°C for 30min (80°C is the radiosynthesized Optimal conditions).
[0098] Radioactive purification:
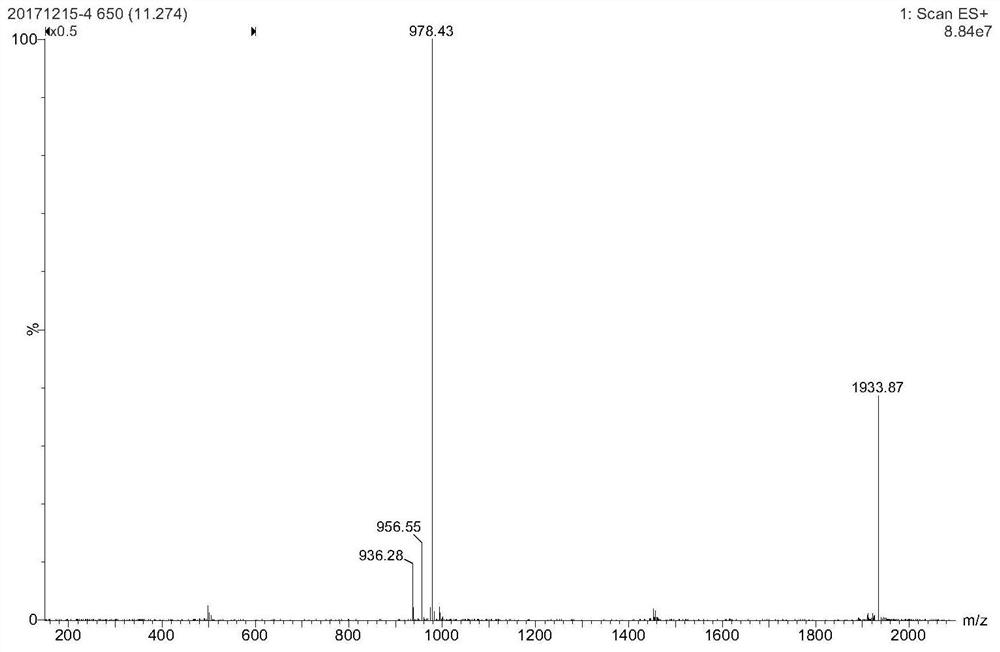
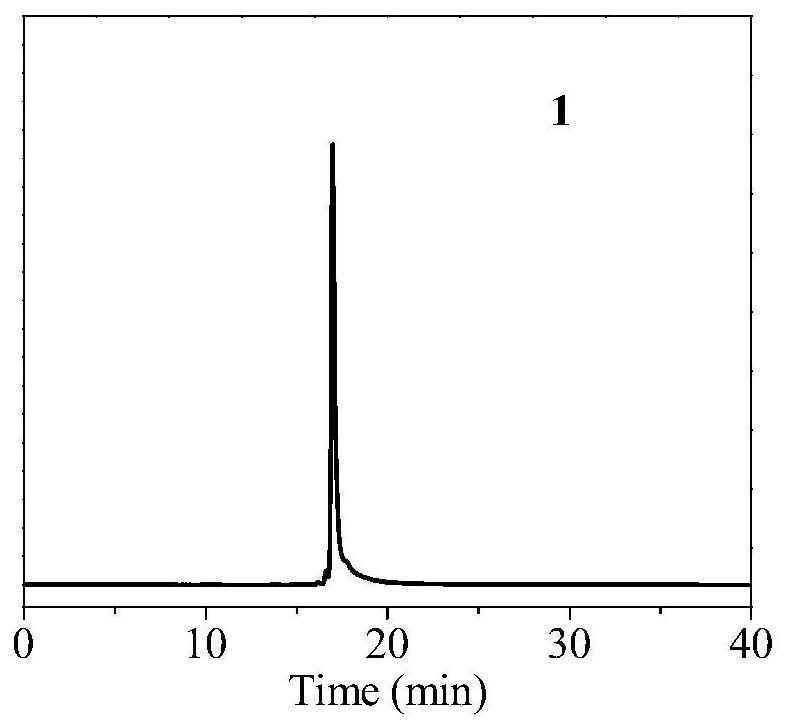
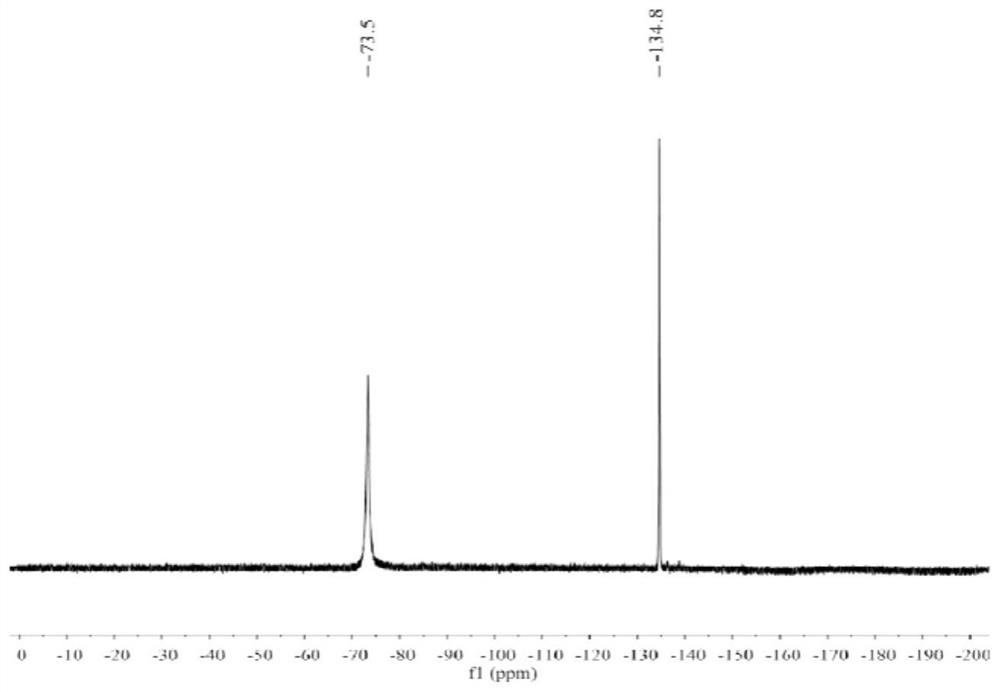
[0099] After the radiosynthesis is complete, transfer the reaction solution to a centrifuge tube containing 20 mL of ultrapure water, and then the resulting radiolabeled probe 18 F-1 was successively loaded on a C18 purification column (colum...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com