An activatable tumor apoptosis pet imaging agent and its preparation method and application
A PET imaging agent and apoptosis technology, which is applied in the field of tumor apoptosis positron emission tomography imaging agent and its preparation, can solve the problems of poor imaging sensitivity, short retention time, and low labeling efficiency, and achieve imaging sensitivity Strong, simple preparation method, short labeling time
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
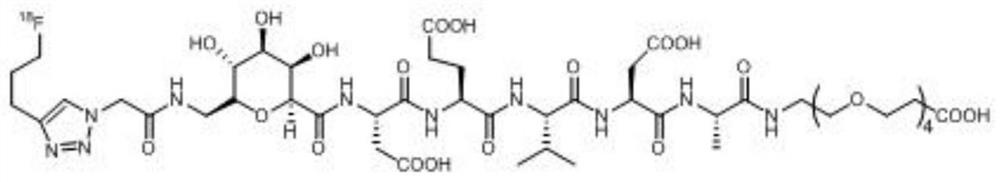
[0062] The DEVD described in this example 18 F marker has a structure shown in the following formula (1):
[0063]
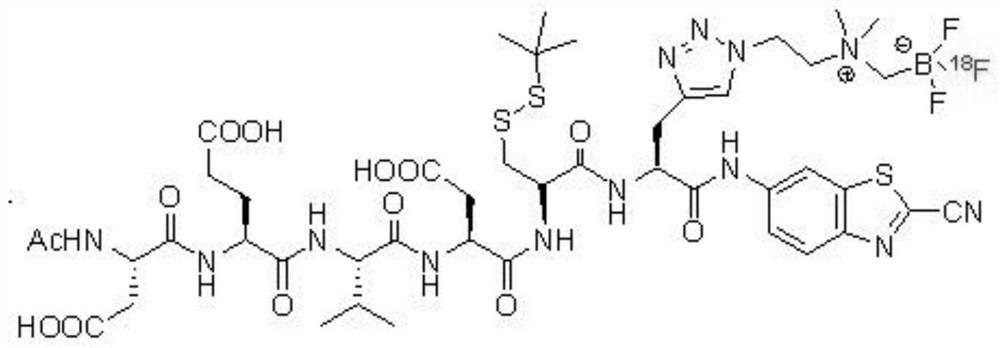
[0064] The DEVD's 18 The synthetic route of the F marker is:
[0065]
[0066] The DEVD's 18 The synthesis steps of F markers include:
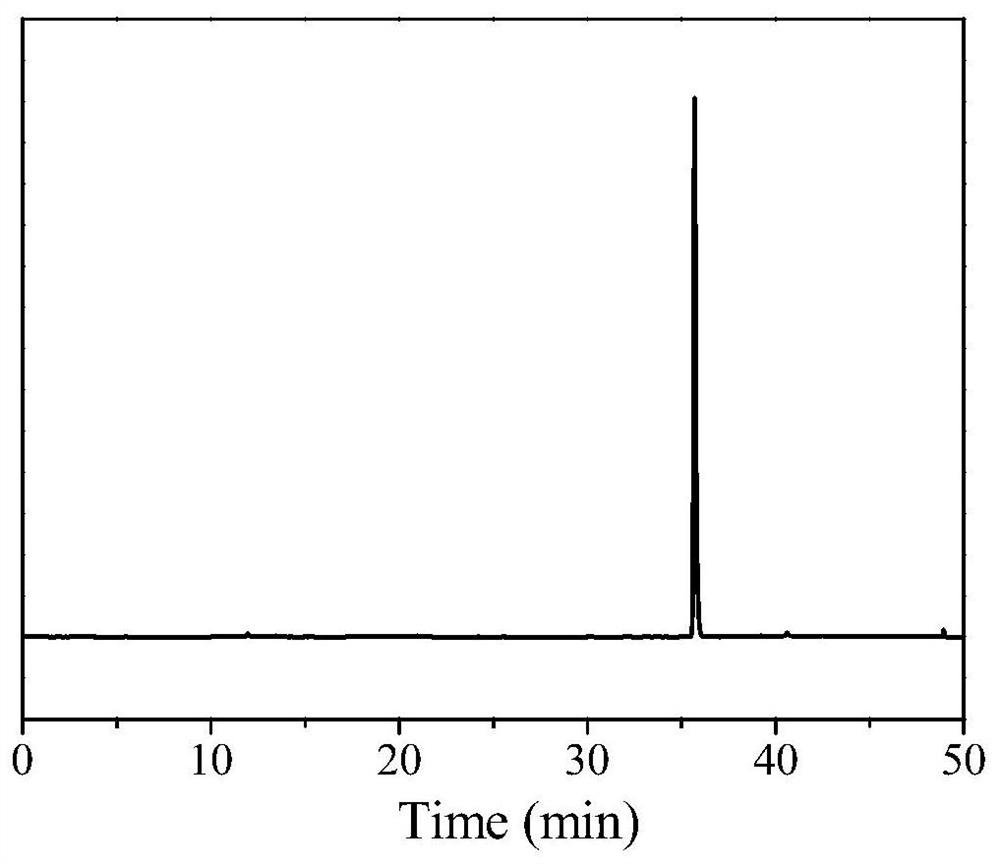
[0067] (1) Add 150 mg (0.325 mmol) of the compound shown in formula (A) and 246 mg (0.380 mmol) of the polypeptide DEVD shown in formula (B) into a 25 mL round bottom flask, add 3 mL of dry tetrahydrofuran (THF) to dissolve, Then add O-benzotriazole-tetramethyluronium hexafluorophosphate (HBTU) 142mg (0.375mmol) and N,N-diisopropylethylamine (DIPEA) 142μL (0.815mmol), under nitrogen protection After reacting at room temperature for 2 hours, the above reaction solution was subjected to TLC detection, developed with dichloromethane:methanol=20:1 (v / v), detected by 254nm ultraviolet light, Rf=0.6, and spin-dried the solvent after judging that the reaction was completed, and obtained the crude product Separation and puri...
Embodiment 2
[0086] The synthesis steps of the 18F marker of the molecular probe DEVD described in this example include:
[0087] (1) Add 150 mg (0.325 mmol) of the compound represented by formula (A) and 243.75 mg (0.325 mmol) of the polypeptide DEVD represented by formula (B) into a 25 mL round bottom flask, add 3 mL of dry tetrahydrofuran (THF) to dissolve , then add O-benzotriazole-tetramethyluronium hexafluorophosphate (HBTU) 147.68mg (0.39mmol) and N, N-diisopropylethylamine (DIPEA) 124.5μL (0.715mmol), nitrogen After reacting at room temperature for 1 h under protection, the above reaction solution was subjected to TLC detection, developed with dichloromethane:methanol=20:1 (v / v), detected by 254nm ultraviolet light, Rf=0.6, and spin-dried the solvent after judging that the reaction was completed, and the The obtained crude product was separated and purified by silica gel column chromatography, using octadecylsilane bonded silica gel as filler, chromatographic column (5 μm, 250 × 4....
Embodiment 3
[0093] The DEVD described in this example 18 The synthesis steps of F markers include:
[0094] (1) Add 150 mg (0.325 mmol) of the compound represented by formula (A) and 252.5 mg (0.390 mmol) of the polypeptide DEVD represented by formula (B) into a 25 mL round bottom flask, add 3 mL of dry tetrahydrofuran (THF) to dissolve , then add O-benzotriazole-tetramethyluronium hexafluorophosphate (HBTU) 123mg (0.325mmol) and N,N-diisopropylethylamine (DIPEA) 152.9μL (0.8775mmol), nitrogen protection After reacting at room temperature for 3 hours, the above reaction solution was subjected to TLC detection, developed with dichloromethane:methanol=20:1 (v / v), detected by 254nm ultraviolet light, Rf=0.5, it was judged that the reaction was complete, and then the solvent was spin-dried, and the The resulting crude product was separated and purified by silica gel column chromatography, using octadecylsilane bonded silica gel as a filler, a chromatographic column (5 μm, 250 × 4.6 mm, Pheno...
PUM
| Property | Measurement | Unit |
|---|---|---|
| purity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com