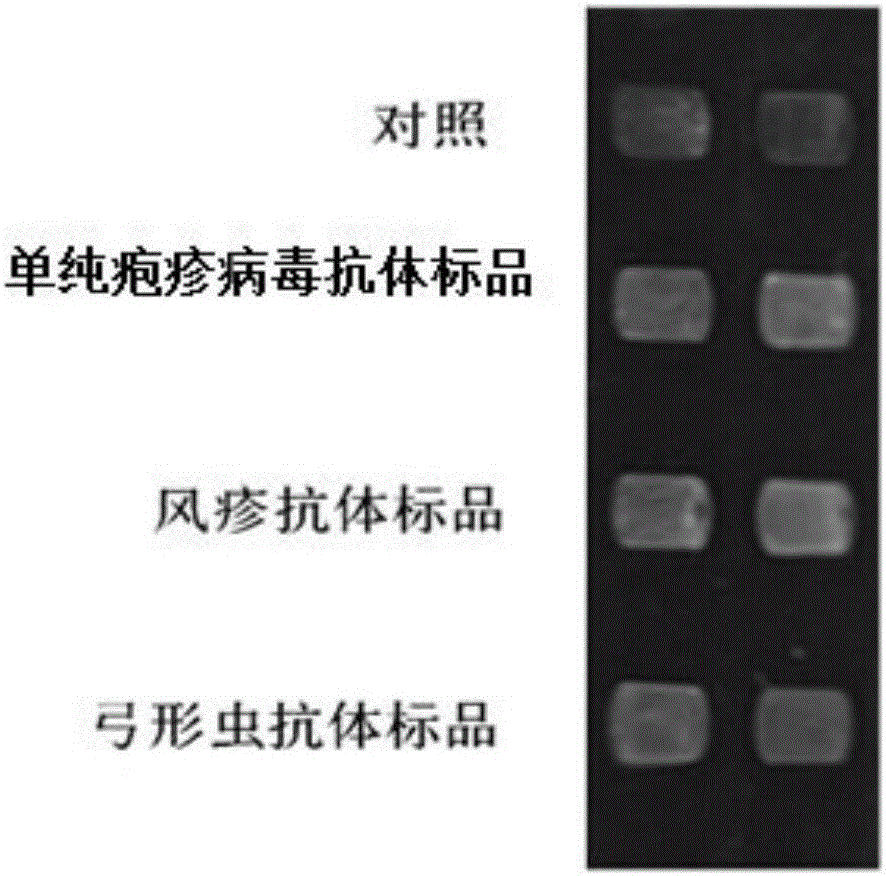
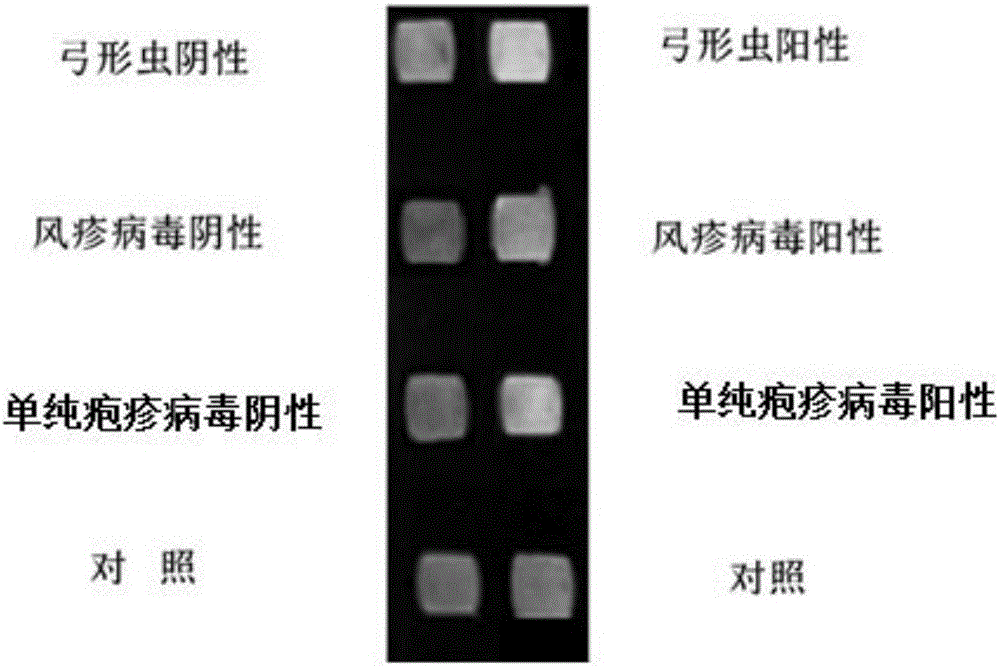
Novel method for identifying toxoplasma, rubella virus and herpes simplex virus at same time
A herpes simplex virus and rubella virus technology, applied in the field of detection and identification, can solve the problems of wrong judgment of pathogens, unclear identification of pathogens, etc., and achieve the effects of accurate results, saving inspection costs, and uniform standards
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0067] A method for simultaneously detecting and / or identifying toxoplasma gondii, rubella virus and herpes simplex virus, comprising the following steps:
[0068] 1. Optimization of silicon wafer surface modification methods:
[0069] This embodiment has adopted a variety of modification methods, and now two kinds of modification methods are screened for optimization. The steps are as follows:
[0070] 1.1 Rinse the wafer with deionized water 3 times, soak the wafer with 1mL of aminosilane and 3mL of concentrated sulfuric acid for half an hour, rinse the wafer with PBS 3 times, soak the wafer with 1mL of glutaraldehyde and 3mL of PBS for 1 hour, and obtain the chemical properties of aldehyde groups on the surface chip.
[0071] 1.2 Rinse the silicon wafer 3 times with deionized water, soak the silicon wafer with 1mL of aminosilane and 3mL of concentrated sulfuric acid for half an hour, rinse the silicon wafer with anhydrous alcohol for 3 times, add saturated sodium succinate...
Embodiment 2
[0122] A protein chip for ellipsometric microscopic imaging, which is prepared by the following method:
[0123] Assembling identification probes on silicon wafers with carboxyl-modified surfaces: assembling the antigens of toxoplasma gondii, rubella virus and herpes simplex virus on the detection sites, and then washing with PBS; then blocking the detection sites with blocking reagents to obtain Ellipsometric microscopic imaging protein chip; preparation parameters refer to the parameters in the standardized detection and / or identification procedures in Example 1.
[0124] Quantitative detection of rubella virus antibody
[0125] 1. Calibration curve and quantitative detection of clinical serum samples
[0126] To establish a calibration curve, serum samples (No. 942, 218 IU / mL) were serially diluted, and the diluted concentration was 0011, 0043, 0170, 0681, and 2725IU / mL5 concentration gradients of rubella virus antibody, diluted with PBST. Rubella virus antigen was immobi...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Sensitivity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com