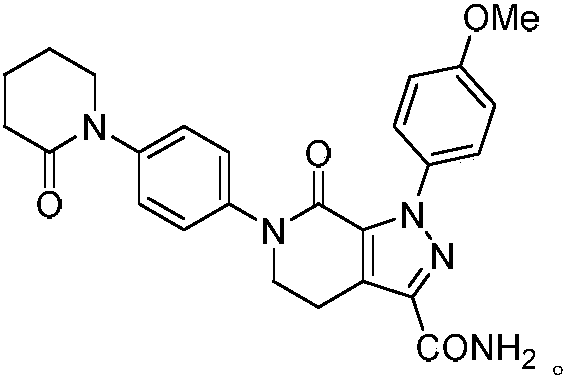
Preparation method of apixaban drug for preventing or treating joint replacement venous thrombosis
A technology of venous thrombosis and apixaban, which is applied in the field of drug synthesis, can solve the problems of cumbersome steps, harsh conditions, and high cost, and achieve the effects of simple process, increased yield, and reduced cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0033] 1-(4-Methoxyphenyl)-7-oxo-6-[4-(2-oxopiperidin-1-yl 3)phenyl]-4,5,6,7-tetrahydro- Preparation of 1H-pyrazole[3,4-c]pyridine-3-carboxylic acid ethyl ester
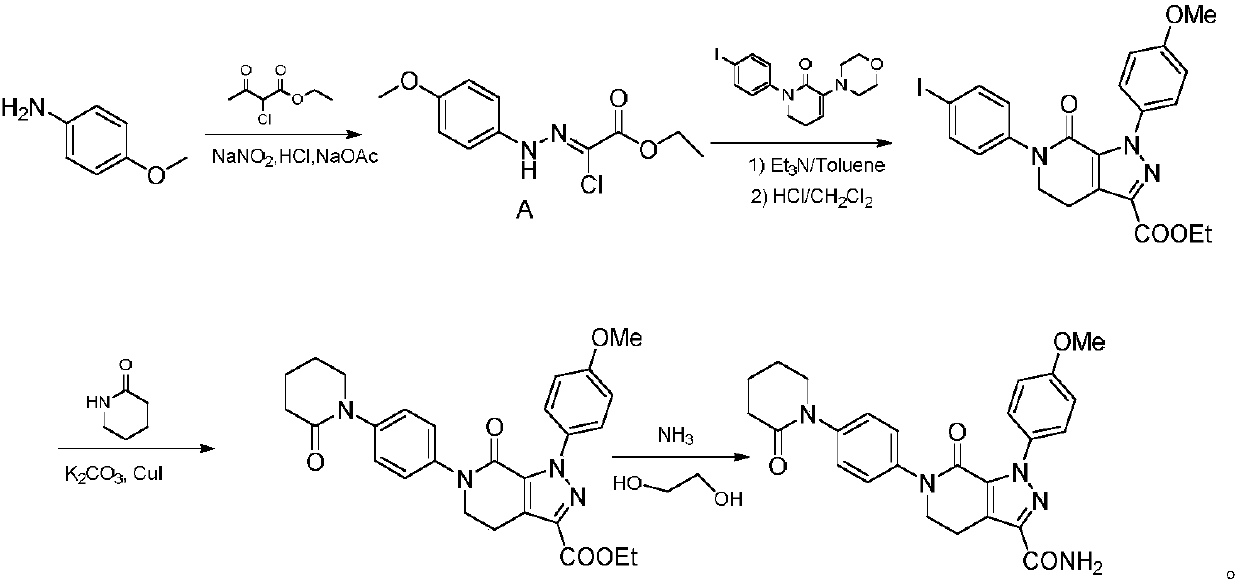
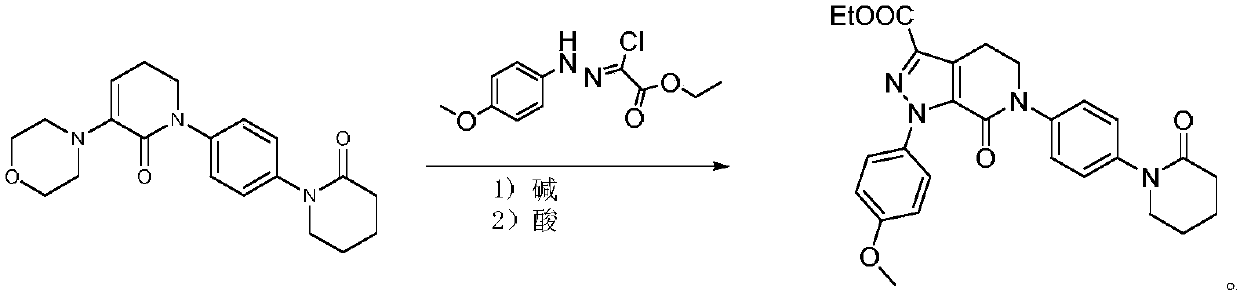
[0034] 1) Under the protection of nitrogen, mix 20.7g (150mmol) of p-methoxyphenylhydrazine, 10.2g (100mmol) of ethyl glyoxylate, 17g (piperidine), 10.2g of molecular sieve and 2.8g (20mmol) of cuprous bromide were added to the flask at 40°C to carry out the catalytic reaction. After the reaction, filter, and add borane dimethyl sulfide complex (2.0M in THF) to the filtrate at 20°C. , Containing borane dimethyl sulfide complex 12.2g) and the compound represented by formula I (DMF solution containing 28.4g of the compound represented by formula I), and then heated to 80°C for stirring and mixing reaction to the formula I The reaction of the compound shown is complete, and the reaction mixture L is obtained;
[0035] 2) The reaction mixture L obtained in step 1) was added to 100ml 4M HCl ice water and stirred for 2.5 hour...
Embodiment 2
[0037] 1-(4-Methoxyphenyl)-7-oxo-6-[4-(2-oxopiperidin-1-yl 3)phenyl]-4,5,6,7-tetrahydro- Preparation of 1H-pyrazole[3,4-c]pyridine-3-carboxylic acid ethyl ester
[0038] 1) Under the protection of nitrogen, p-methoxyphenylhydrazine 19.3g (140mmol), ethyl glyoxylate 10.2g (100mmol), alkali 25.5g (piperidine), Molecular sieve 8.2g and cuprous bromide 1.4g (10mmol) were added to the flask at 35°C for catalytic reaction. After the reaction was completed, filtered, and added borane dimethyl sulfide complex (2.0M in THF) to the filtrate at 20°C. , Containing borane dimethyl sulfide complex 13.7g) and the compound represented by formula I (DMF solution containing 24.9g of the compound represented by formula I), and then heated to 60°C for stirring and mixing reaction to the formula I The reaction of the compound shown is complete, and the reaction mixture L is obtained;
[0039] 2) The reaction mixture L obtained in step 1) was added to 100ml 4M HCl ice water and stirred for 2.5 hours, ...
Embodiment 3
[0041] 1-(4-Methoxyphenyl)-7-oxo-6-[4-(2-oxopiperidin-1-yl 3)phenyl]-4,5,6,7-tetrahydro- Preparation of 1H-pyrazole[3,4-c]pyridine-3-carboxylic acid ethyl ester
[0042] 1) Under the protection of nitrogen, 16.6g (120mmol) of p-methoxyphenylhydrazine, 10.2g (100mmol) of ethyl glyoxylate, 17.8g (tetrahydropyrrole), 13.3g of molecular sieve and 2.2g (15mmol) of cuprous bromide were added to the flask at 45°C to carry out the catalytic reaction. After the reaction was completed, it was filtered, and the borane dimethyl sulfide complex (2.0M in THF) was added to the filtrate at 25°C. , Containing borane dimethyl sulfide complex 11.4g) and the compound represented by formula I (DMF solution containing 31.9g of compound represented by formula I), and then heated to 70°C for stirring and mixing reaction to the formula I The reaction of the compound shown is complete, and the reaction mixture L is obtained;
[0043] 2) The reaction mixture L obtained in step 1) was added to 100ml 4M HCl ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com