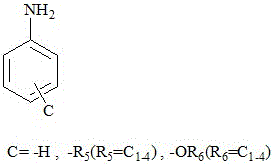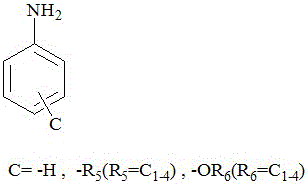Preparation method of N-substituted pyridone pyridine quaternary ammonium salt monoazo dye
A pyridone-based pyridine, monoazo dye technology, applied in monoazo dyes, azo dyes, organic dyes and other directions, can solve the problems of decreased light fastness, etc., and achieve high light fastness and color strength. High, excellent dyeing performance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0023] (Dye structure A: 3-CH 3 ,B:-CH 2 CH 2 CH 2 NMe 2 ,C:H)
[0024] Dissolve 1.88 g of aniline in 15 mL of water, slowly add 5 mL of concentrated hydrochloric acid dropwise to the water, cool down to 0-2 °C, add dropwise a solution composed of 1.45 g of sodium nitrite and 3 mL of water while controlling the temperature at 0-5 °C, and the dropwise addition is completed Finally, the excess sodium nitrite is destroyed with 10% sulfamic acid, and the diazonium salt is ready for use. Take 25.0g of pyridone solution and add it dropwise to the diazonium salt solution at 0-10°C for 20 minutes, stir and react for 1 hour after the dropwise addition is completed, adjust the pH to 3-5 with sodium hydroxide solution, discharge the material and bottle it. A red liquid was obtained, and the dyed paper was orange-yellow.
Embodiment 2
[0026] (Dye structure A: 3-CH 3 ,B:-CH 2 CH 2 CH 2 NMe 2 , C: 4-CH 3 )
[0027] Take p-toluidine as raw material to carry out experiment, other conditions are with embodiment 1. A red liquid was obtained, and the dyed paper was orange-yellow.
Embodiment 3
[0029] (Dye structure A: 3-CH 3 ,B:-CH 2 CH 2 CH 2 NMe 2 , C: 3-CH 3 -2-OCH 3 )
[0030] Synthesis experiments were carried out using crixidin as a raw material, and other conditions were the same as in Example 1. A black liquid was obtained, and the paper was orange-red.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com