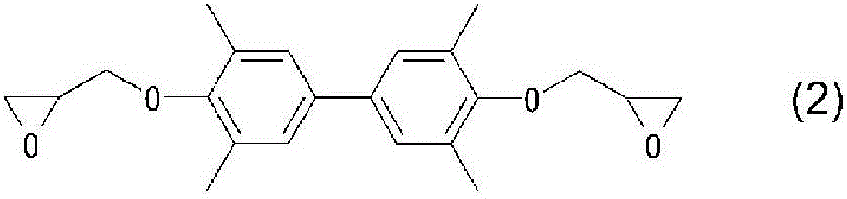
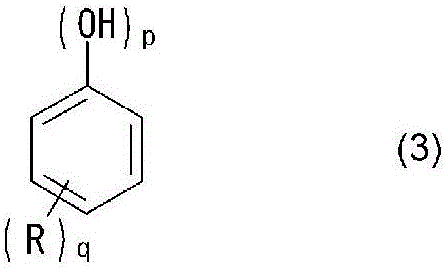Phenolic resin, epoxy resin containing the phenolic resin, cured product of the epoxy resin composition, and semiconductor device having the cured product
A technology of phenolic resin and epoxy resin, which is applied in the direction of semiconductor devices, semiconductor/solid-state device components, electric solid-state devices, etc., can solve problems such as substrate material warpage, achieve high thermal shrinkage rate, reduce warpage, and thermal expansion rate high effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
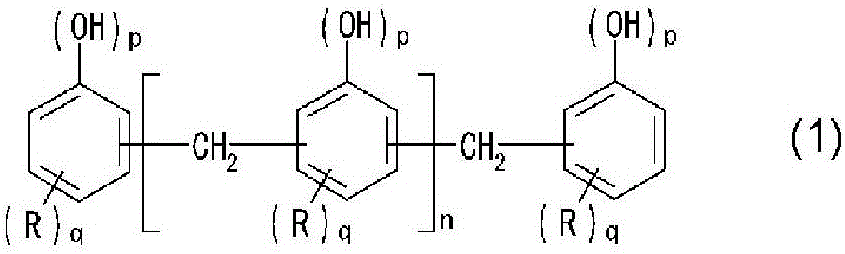
[0107] Add 134 parts (1.0 mol) of o-allylphenol, 32 parts (0.98 mol) of 92% paraformaldehyde, pure 0.4 parts of water and 1.1 parts of oxalic acid. After reacting at 100°C for 12 hours under reflux and further reacting at 160°C for 2 hours, it was cooled to 95°C. After cooling, 130 parts of pure water of 90° C. or higher were thrown in and washed with water. Thereafter, by raising the internal temperature to 160° C. and performing a decompression-steaming treatment, unreacted components were removed to obtain a novolac resin A (R in general formula (1) is an allyl group, p=1 , q=1 novolac resin). The obtained novolac resin A had a softening point of 73° C., a melt viscosity at 150° C. of 4.3P, a hydroxyl equivalent of 170 g / eq, and a gelation time of 72 seconds. The compound of n=0 measured by gel permeation chromatography was 5.9 area % of the whole phenolic resin, and the compound of n=1 was 6.2 area % of the whole phenolic resin.
Embodiment 2
[0109] Add 134 parts (1.0 moles) of o-allylphenol, 36 parts (1.1 moles) of 92% paraformaldehyde, pure 0.4 parts of water and 1.1 parts of oxalic acid. After reacting at 100°C for 12 hours under reflux and further reacting at 160°C for 2 hours, it was cooled to 95°C. After cooling, 130 parts of pure water of 90° C. or higher were thrown in and washed with water. Thereafter, by raising the internal temperature to 160° C. and performing a decompression-steaming treatment, unreacted components were removed to obtain a novolak resin B (R in general formula (1) is an allyl group, p=1 , q=1 novolac resin). The obtained novolak resin B had a softening point of 98°C, a melt viscosity at 150°C of 20P, and a hydroxyl equivalent of 172 g / eq.
Embodiment 3
[0111]In a glass flask with a capacity of 2000 parts equipped with a thermometer, a feed / distillation outlet, a cooler and a stirrer, 1200 parts (9.0 moles) of o-allylphenol, 127 parts (1.8 moles) of 42% formalin, And 12 parts of oxalic acid. The reaction was carried out at 100° C. for 7 hours under reflux. After completion of the reaction, 600 parts of pure water of 90° C. or higher were thrown in and washed with water. Thereafter, by raising the internal temperature to 160° C. and performing a decompression-steaming treatment, unreacted components were removed to obtain a novolac resin C (R in the general formula (1) is an allyl group, p=1 , q=1 novolac resin). The obtained novolak resin C was liquid at normal temperature, and had a hydroxyl equivalent of 141 g / eq.
PUM
| Property | Measurement | Unit |
|---|---|---|
| elastic modulus | aaaaa | aaaaa |
| softening point | aaaaa | aaaaa |
| epoxy equivalent | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com