A kind of preparation method of trihydroxyisoflavone
A technology of trihydroxyisoflavone and dimethoxyisoflavone, which is applied in the field of preparation of trihydroxyisoflavone, can solve the problems of no economical and efficient method, few activity research reports, expensive raw materials, etc., and achieves easy access , Simplify industrial operation, low cost effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
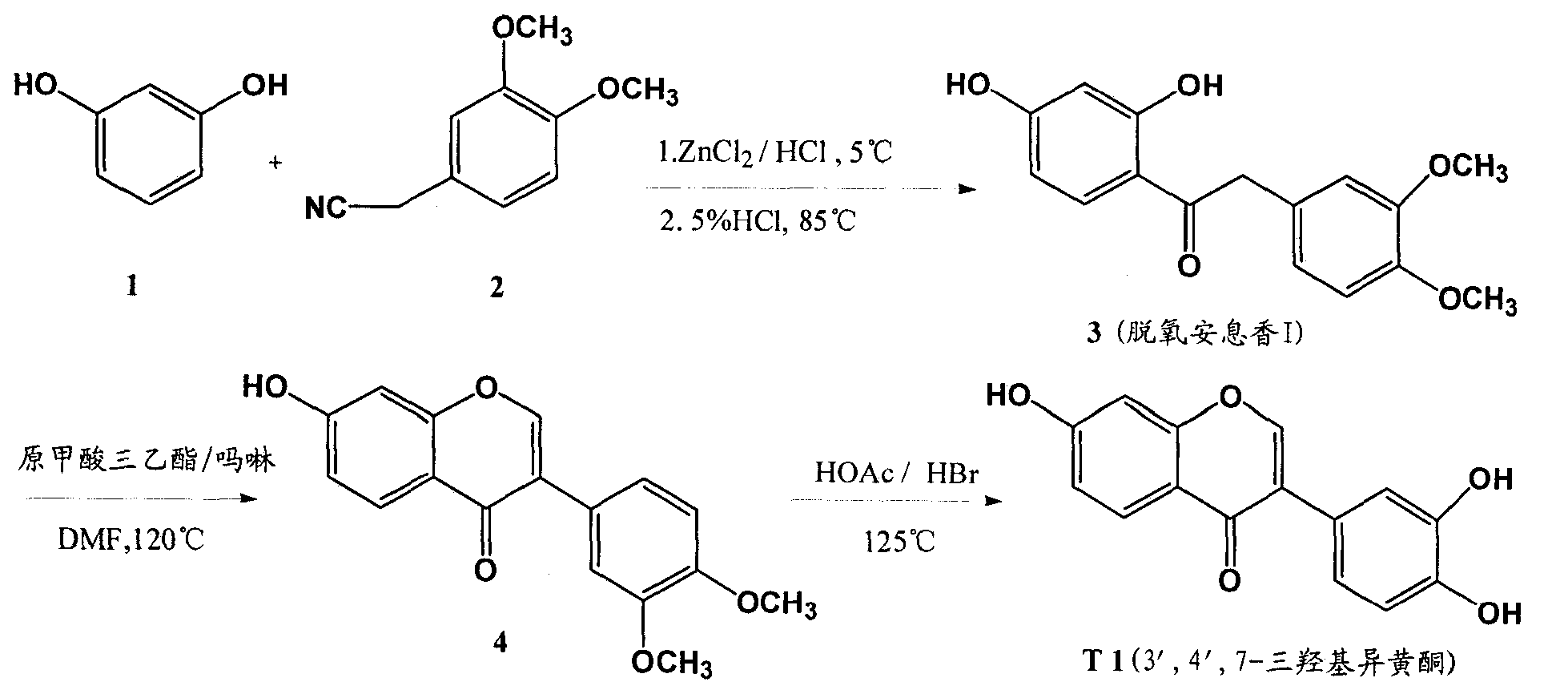
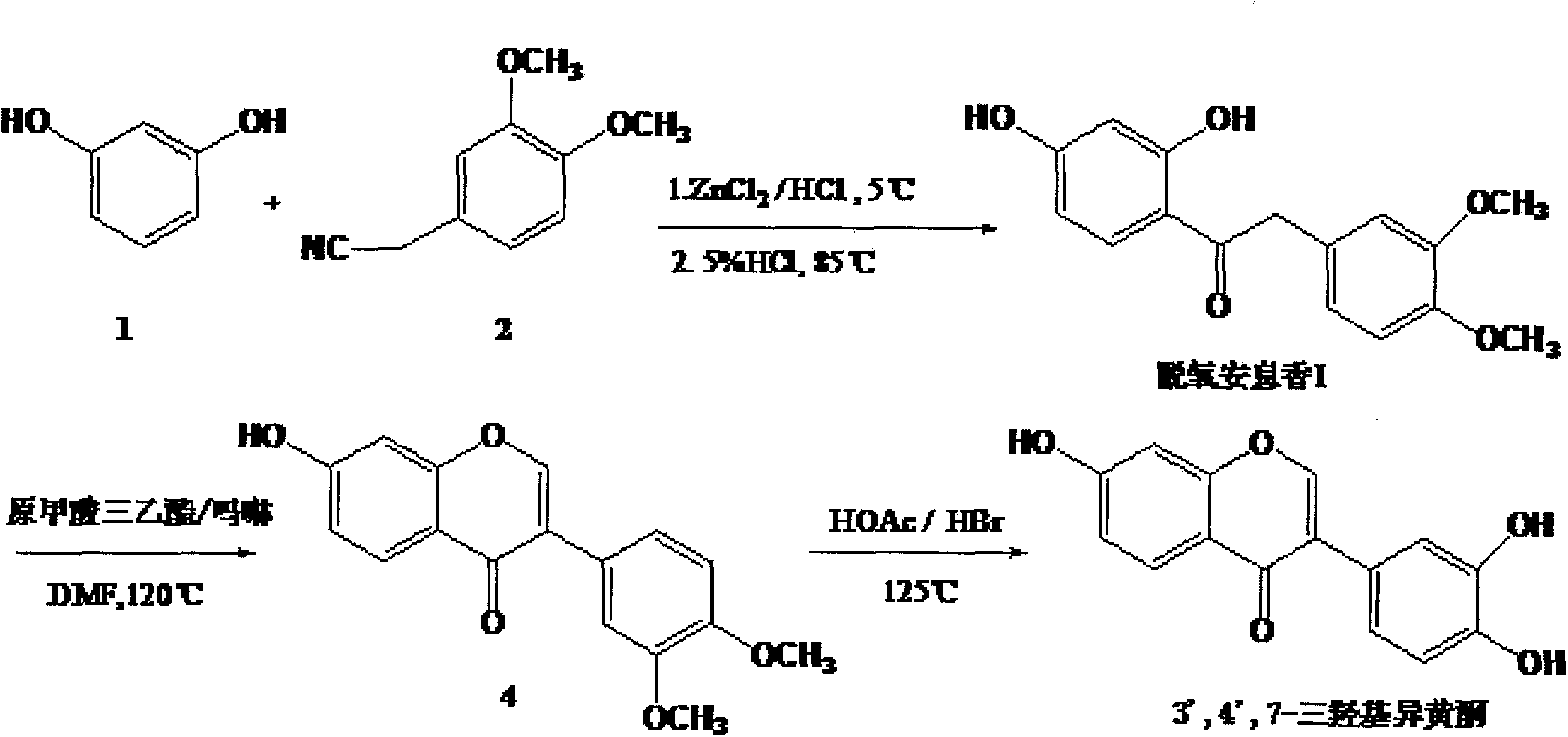
[0038] Embodiment 1 prepares 3 ', 4 ', 7-trihydroxy isoflavone (T1)
[0039] 1-1 Preparation of deoxybenzoin I (compound 3)
[0040] Add 2.5 L of isopropyl ether and 400 g of 3,4-dimethoxyphenylacetonitrile into the reaction flask, stir and dissolve with strong mechanical force, then add 278 g of anhydrous zinc chloride at 0-5°C, and continuously feed dry hydrogen chloride gas to The solution in the reaction flask was saturated, 245 g of resorcinol was added, the temperature was maintained at 0-5°C, and the reaction was carried out with airtight stirring for 12 hours. After standing still, add 5L of 5% dilute hydrochloric acid aqueous solution, raise the temperature to 85°C, stop heating after TLC detects that the reaction is complete, cool to room temperature, a large amount of yellow solid precipitates, filter under reduced pressure, wash the filter residue with water until neutral, recrystallize with ethanol , to obtain a pale yellow-white solid powder, dried to constant w...
Embodiment 2
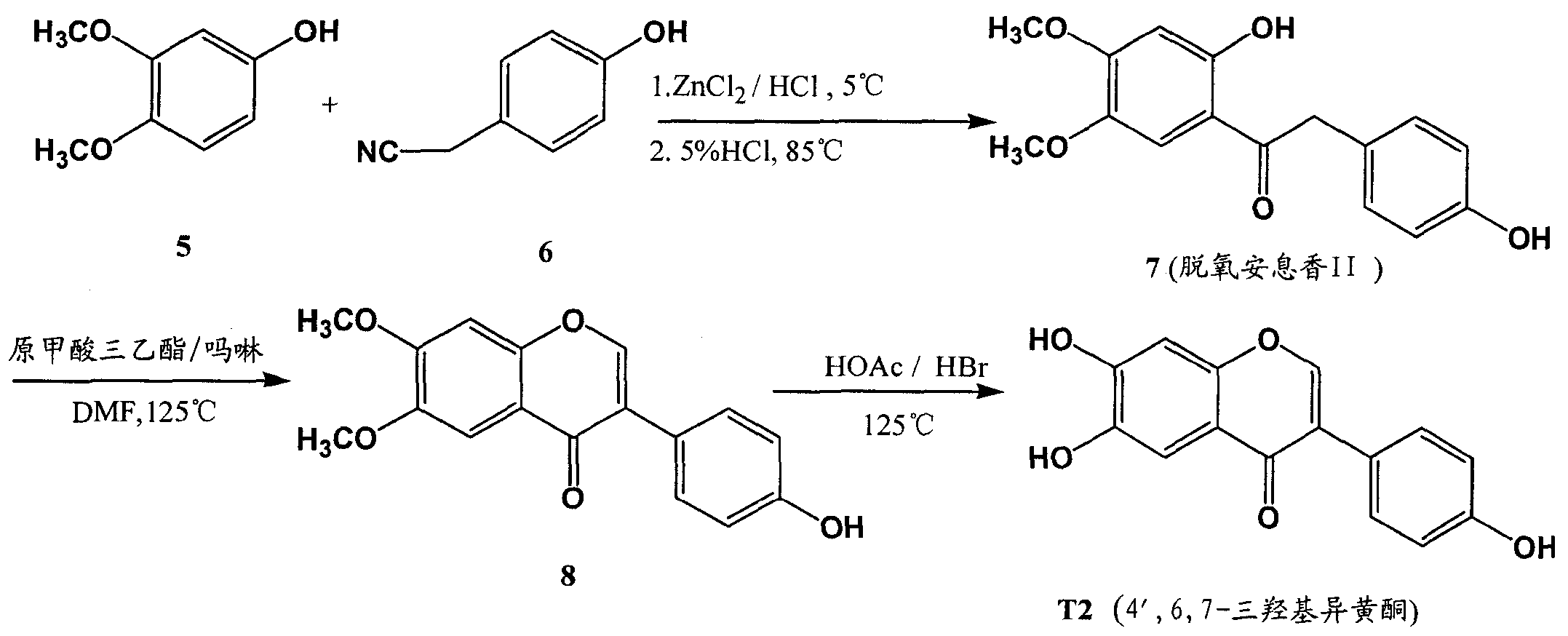
[0045] Example 2 Preparation of 4', 6, 7-trihydroxyisoflavones (T2)
[0046] 2-1 Preparation of deoxybenzoin II (compound 7)
[0047] Add 2L of isopropyl ether and 240g of p-hydroxyphenylacetonitrile into the reaction flask. After stirring and dissolving with strong mechanical force, add 170g of anhydrous zinc chloride at 0-5°C, continuously feed dry hydrogen chloride gas to saturation, and add 3,4- 200g of dimethoxyphenol, keep at 0-5°C, and keep it under closed stirring for 10h, then add 4L of 5% dilute hydrochloric acid aqueous solution, heat up to 85°C, after TLC detects that the reaction is complete, stop heating, cool to room temperature, and a large amount of yellow solid precipitates . Filtrate under reduced pressure, wash the filter residue with water until neutral, and recrystallize from absolute ethanol to obtain 252 g of deoxybenzoin II (compound 7), which is a light yellow-white solid powder with a yield of 53.8%, mp: 177.6-178.9°C. 1 H NMR (400MHz, DMSO) δppm: ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com