Bufadienolide compound and application thereof to preparation of medicine for resisting gastric cancer
A technology of bufadienolide and compound is applied in the field of anti-gastric cancer drugs, which can solve the problems of unreported anti-gastric cancer activity of bufadienolide compounds.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0014] Example 1: Establishment of a 3D cell spheroid screening model for gastric cancer
[0015] Sand toad toxin, sand toad toxin-3-pimeloyl arginine ester, far cinobufin toxin-3-adipyl arginine ester, far cinobufin toxin, deacetylated cinobufin toxin, Chinese Bufafen-3-suberoyl arginine ester, bufafen-3-suberoyl arginine ester, 3-dehydrobufafolin and sea bufo toxin were sourced from Shanghai Yuanye Biotechnology Co., Ltd. .
[0016] Docetaxel and epirubicin hydrochloride were purchased from Dalian Meilun Biotechnology Co., Ltd.; gastric cancer cell line HGC-27 was purchased from Shanghai Cell Bank, Chinese Academy of Sciences; ULA-96-well plates were purchased from Corning; inverted microscopes were purchased from OLYMPUS.
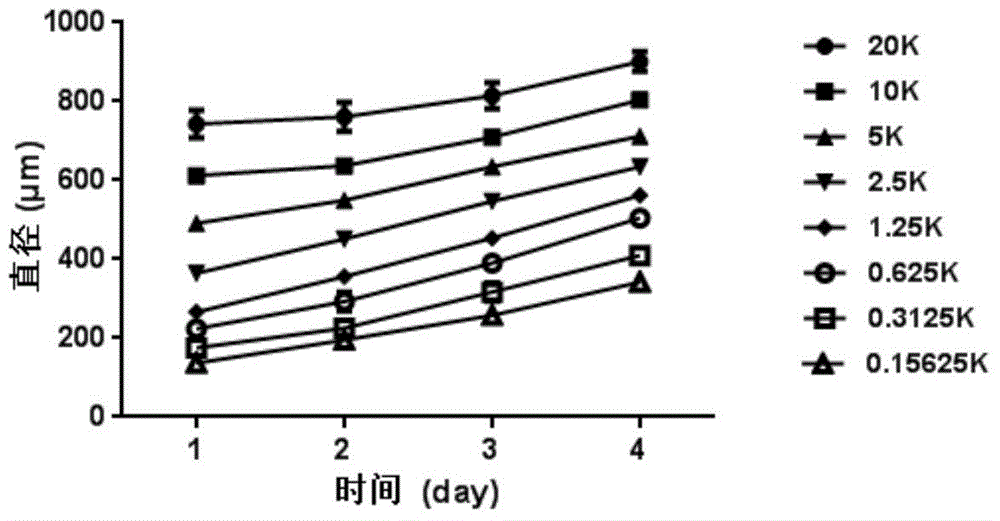
[0017] The HGC-27 cells in the logarithmic growth phase were seeded in ULA-96 well plates at a density of 20000 cells / well (20K cells / well), 10000 cells / well, 5000 cells / well, 2500 cells / well, and 1250 cells / well. Cells / well, 625 cells / well, 313 cells / ...
Embodiment 2
[0018] Example 2: Inhibitory effect of coarsely screened bufadienolactone compounds on the growth of 3D cell spheres
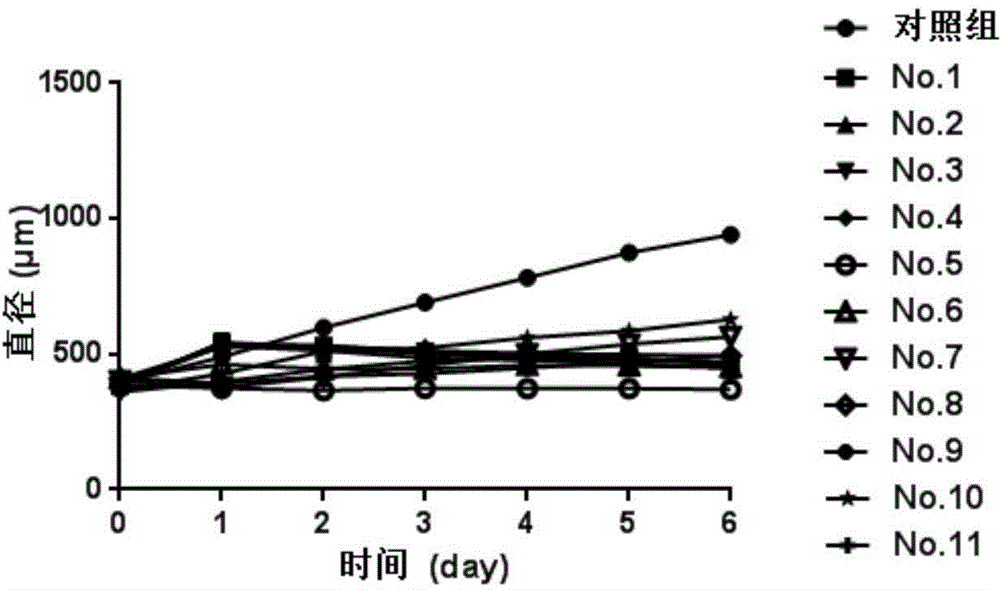
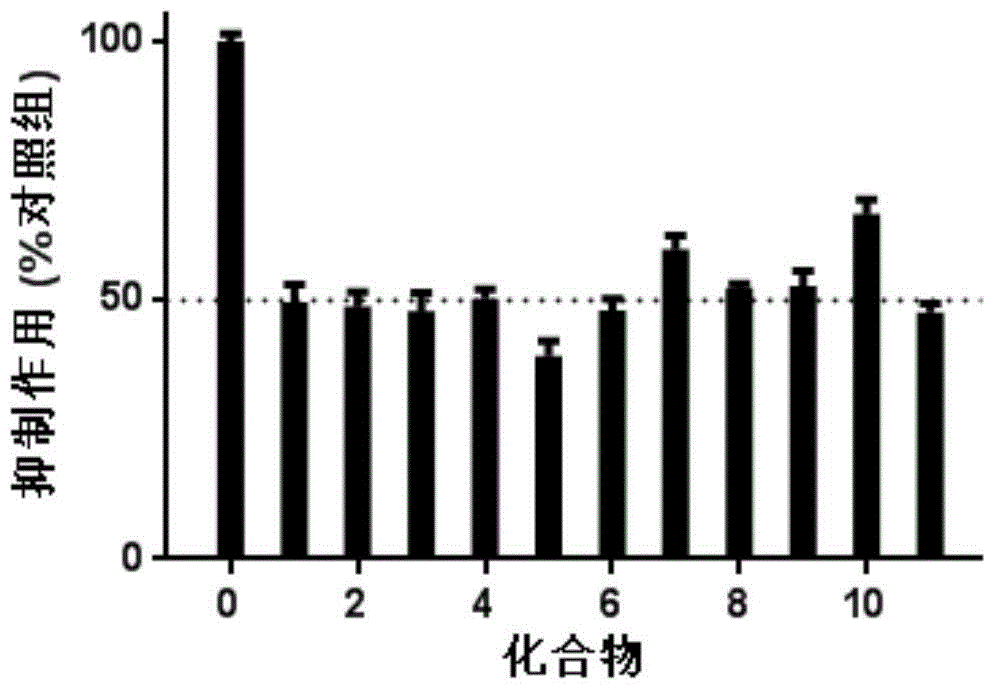
[0019] Docetaxel and epirubicin hydrochloride, typical drugs for the clinical treatment of gastric cancer, were selected as positive control drugs, and the inhibitory effect of 9 typical bufadiene lactone compounds on the growth of HGC-27 3D cell spheres was roughly screened. Among them, 9 typical bufadiene lactone compounds (as a coarse screening drug) are bufabufagin, bufabufalin-3-pimeloyl arginate, and bufabufalin-3-adipyl Arginine Esters, Cinobufagin, Deacetylated Cinobufagin, Cinobufagin-3-Suberoyl Arginate, Cinobufagin-3-Suberoyl Arginate, 3 - Dehydrobufalin and sea bufotoxin. Cells at the optimal density (313 cells / well) were inoculated on ULA-96 well plates, and the inoculation volume of the medium (RPMI-1640 containing 20% fetal bovine serum) was 200 μL / well. After culturing for 4 days and growing to 3D cell spheres with optimal particle size, 10...
Embodiment 3
[0020] Example 3: The dose relationship of deacetylated cinobufagin inhibiting the growth of 3D cell spheres
[0021] Different doses of the compound with the strongest inhibitory ability, 5-deacetylcinobufagin, were applied to the 3D cell spheres. Inoculate the cells with optimal density (313 cells / well) in ULA-96 well plate, culture medium (RPMI-1640 containing 20% fetal calf serum) with an inoculation volume of 200 μL / well for 4 days, and grow to the optimal particle size After 3D cell spheres, replace 100 μL of fresh medium in each well, add compound 5 to 16 wells with a volume of 10 μL, and the concentration gradient of the compound is 40 μM, 20 μM, 10 μM, 5 μM, 2.5 μM, 1.25 μM, 0.625 μM and 0.3125 μM, each concentration was replicated twice. The blank control group was cell spheres treated with medium containing 0.2% DMSO (RPMI-1640 containing 20% fetal bovine serum), and two wells were used as the blank control group. Use an inverted microscope to record the size ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com