Tulathromycin related substance, enriching and preparation method and application thereof
A technology of telamycin and related substances, applied in the field of telamycin-related substances, can solve the problems of lack of telamycin separation, detection methods, inability to identify and confirm the structure, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0094] 5mg tyramycin bulk drug was dissolved in 1mL acetonitrile, and the injection volume was 20μL.
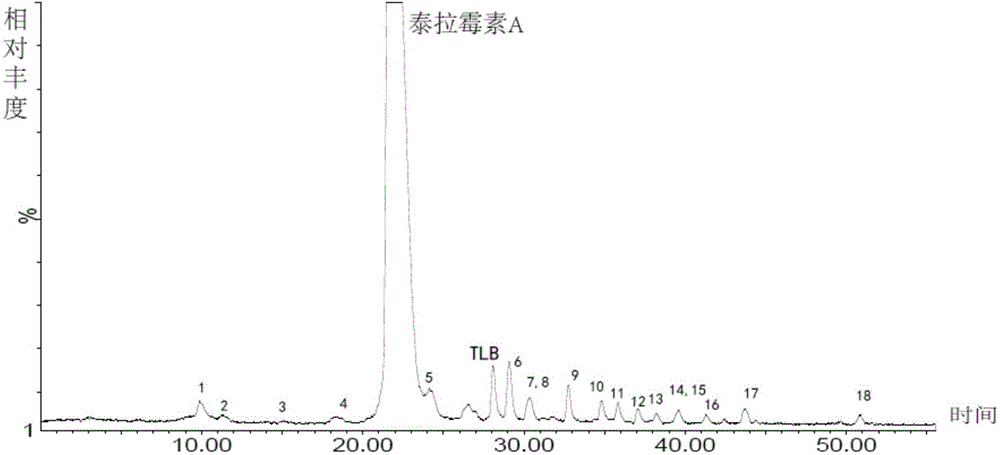
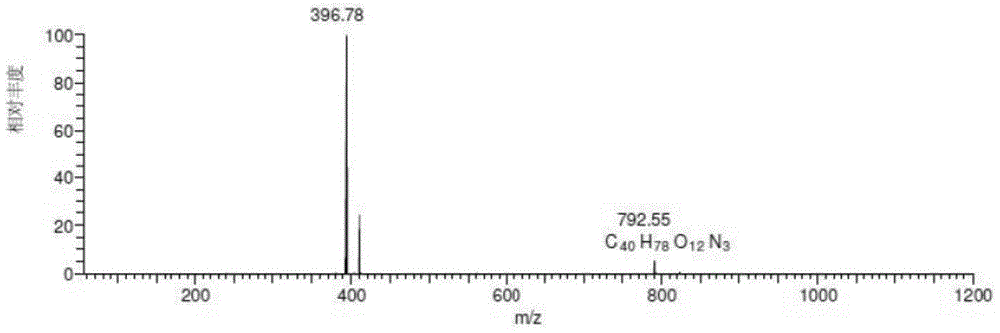
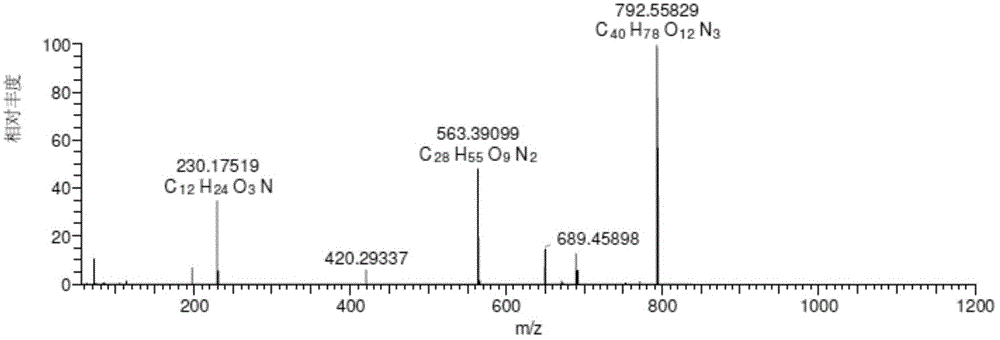
[0095] Chromatographic parameters: Xbridge TM C 18 (250*4.6mm, 5μm) column, mobile phase A: 0.35% formic acid aqueous solution (ammonia water adjusted to pH 7.66), mobile phase B: methanol: acetonitrile = 45:25, gradient elution 0→15min, A:B=65 :35; 15→40min, A:B=65:35→30:70; 40→55min, A:B=30:70, UV absorption wavelength 205nm, column temperature 35℃, flow rate 1.0ml / min, HPLC fraction After passing through the UV detector, it enters the MS detection with a 3:1 split. The total ion current of the mass spectrometer is as figure 1 As shown, the impurities are shown in Table 1. When the mobile phase salt concentration is 0.35%, the main peak retention time is appropriate and the peak shape is good. Related substances can be well separated from the main peak. It can be seen from the figure that the method of the present invention can be used to detect teramycin and its impuriti...
Embodiment 2
[0105] 5mg tyramycin crude drug was dissolved in 1mL acetonitrile, and the injection volume was 20μL.
[0106] Chromatographic parameters: Xbridge TM C 18 (250*4.6mm, 5μm) column, mobile phase A: 0.3% formic acid aqueous solution (ammonia water adjusted to pH 7.66), mobile phase B: methanol: acetonitrile=45:25, gradient elution 0→15min, A:B=65 :35; 15→40min, A:B=65:35→30:70; 40→55min, A:B=30:70, UV absorption wavelength 205nm, column temperature 35℃, flow rate 1.0ml / min, HPLC fraction After passing through the UV detector, it enters the MS detection with a 3:1 split. The total ion current of the mass spectrometer is as Image 6 Shown. When the mobile phase salt concentration is 0.3%, the main peak retention time is appropriate and the peak shape is good. Related substances can be well separated from the main peak.
Embodiment 3
[0108] 5mg tyramycin crude drug was dissolved in 1mL acetonitrile, and the injection volume was 20μL.
[0109] Chromatographic parameters: Xbridge TM C 18 (250*4.6mm, 5μm) column, mobile phase A: 0.4% formic acid aqueous solution (ammonia water to adjust the pH value of 7.66), mobile phase B: methanol: acetonitrile = 45:25, gradient elution 0→15min, A:B=65 :35; 15→40min, A:B=65:35→30:70; 40→55min, A:B=30:70, UV absorption wavelength 205nm, column temperature 35℃, flow rate 1.0ml / min, HPLC fraction After passing through the UV detector, it enters the MS detection with a 3:1 split. The total ion current of the mass spectrometer is as Figure 7 Shown. When the mobile phase salt concentration is 0.4%, the main peak retention time is appropriate and the peak shape is good. Related substances can be well separated from the main peak.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Concentration | aaaaa | aaaaa |
| Uv absorption wavelength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com