Preparation method for novel cepharanthine and application of cepharanthine in pharmaceuticals
A technology of Stephalin and its chemical structural formula, which is applied in the preparation of new Stephalin and its application in medicine
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
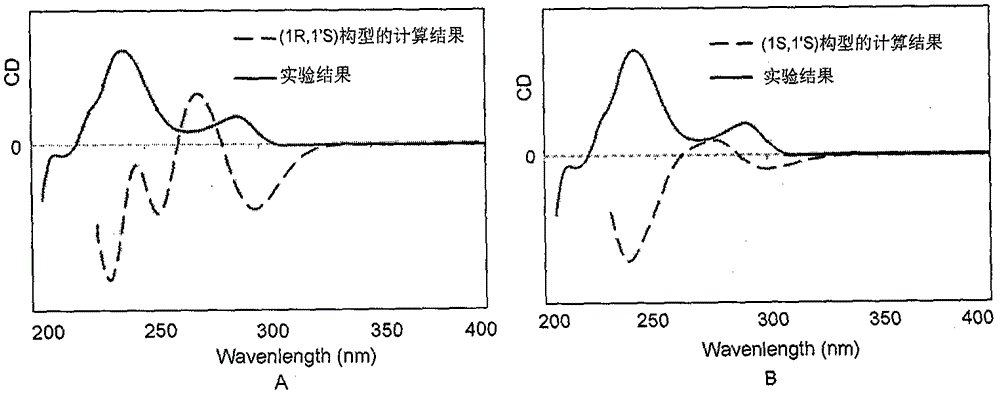
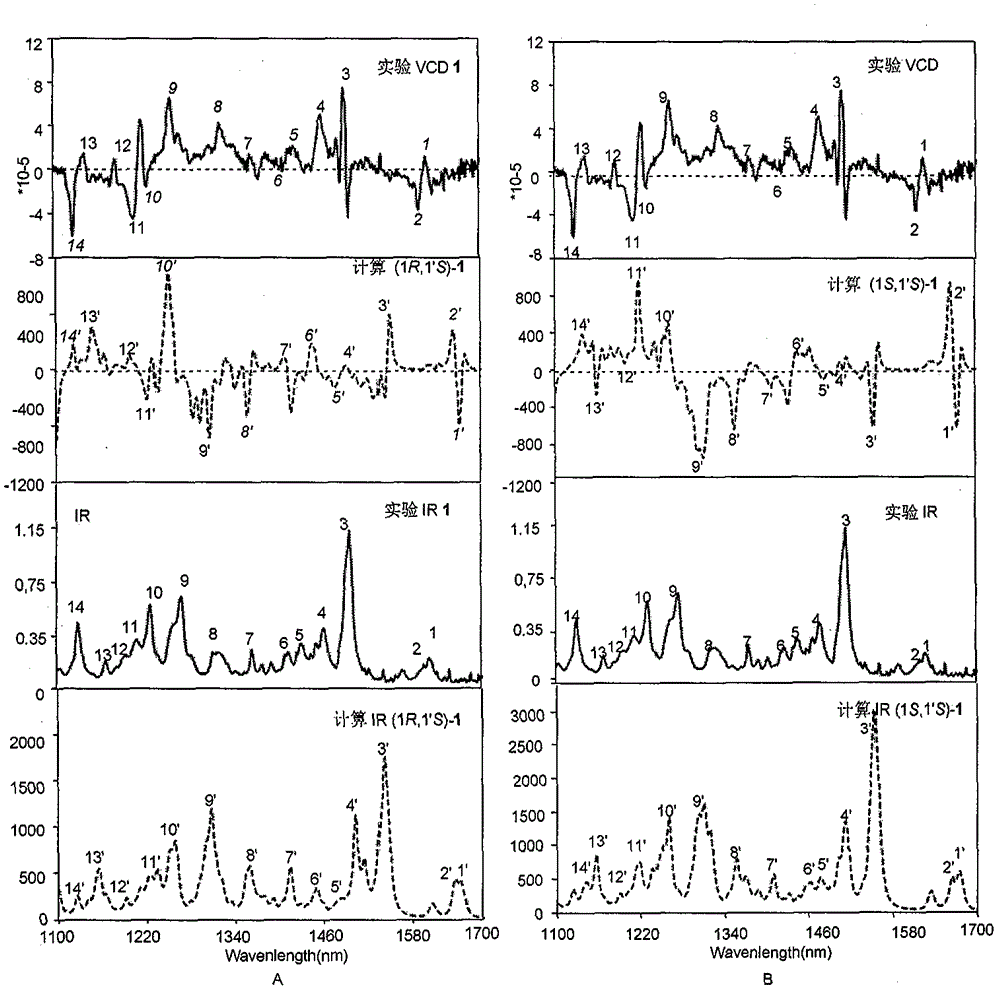
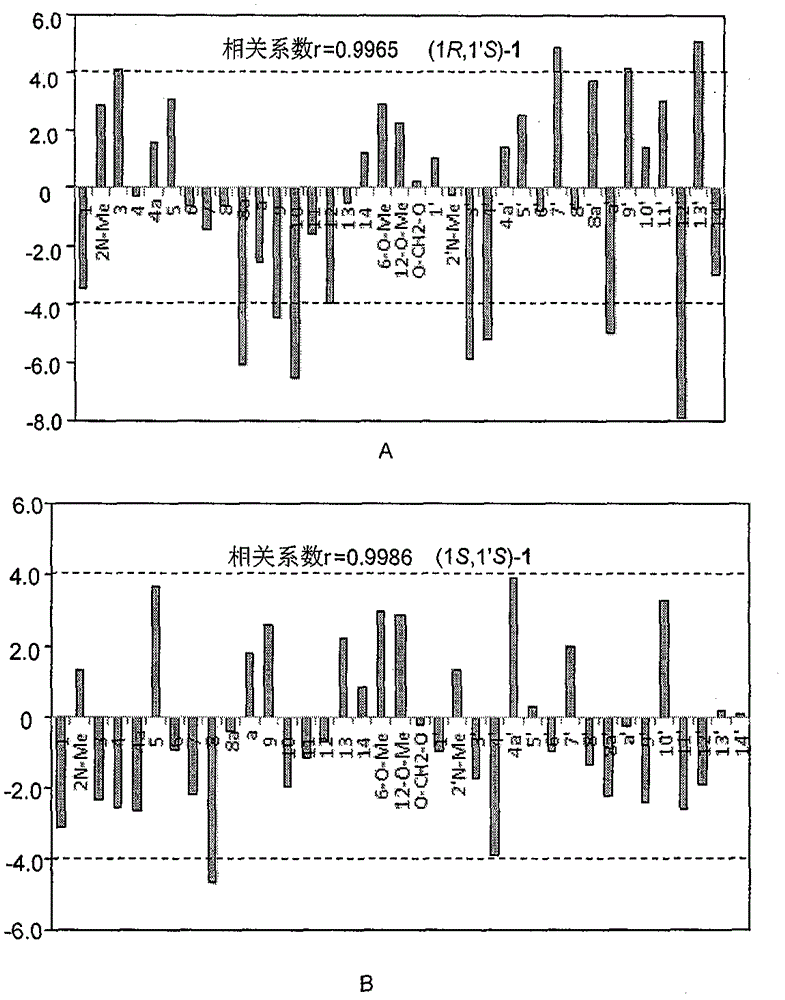
Image
Examples
Embodiment 1
[0018] Extraction and preparation of (1R, 1′R)-stephalin:
[0019] Purchase 5 kg of root tubers from Anguo Traditional Chinese Medicine Market, crush them, extract 3 times with an appropriate amount of 80% alcohol, concentrate under reduced pressure at 45°C to obtain 80 g of extracted crude extract, dissolve the crude extract in water, and extract 5 times with appropriate amount of ethyl acetate , to obtain extract 50g. The extract was mixed with 200-300 mesh silica gel, then eluted with petroleum ether / ethyl acetate (4:1, 3:2, 2:3, 1:4, pure ethyl acetate) to obtain Fr.1 - 5 components, component Fr.3 was repeatedly separated by silica gel chromatography column, Sephadex LH-20 gel chromatography column, and RP-C18 reverse-phase packing chromatography column to obtain 10 mg of the target compound.
Embodiment 2
[0021] Identification of (1R, 1′R)-stephalin structure:
[0022] Qualitative analysis data and collection of illustrative plates of the compound prepared in embodiment 1:
[0023] 1 H NMR (600MHz, CDCl 3 )δ: 2.38-2.44, 2.63-2.68 (2m, 2×2H, 2×NCH 2 ), 2.56(s, 3H, N-Me), 2.64(s, 3H, N-Me), 2.76-2.79, 2.84-2.87(m, 2×2H, 2×CH 2 ), 3.60(s, 1H, CH), 3.68(s, 3H, OMe), 2.98-3.31(m, 4H, 2×CH 2 ), 3.88(s, 3H, OMe), 4.17(s, 1H, CH), 5.59, 5.55(ds, 2H, OCH 2 O), 5.47(s, 1H, C 6 h 3 ), 6.32(s, 1H, C 6 h 2 ), 6.64(s, 1H, C 6 h 2 ), 6.77 (ds, 2H, C 6 h 3 ), 6.35(s, 1H, C 6 h 1 ), 7.03(s, 1H, C 6 h 4 ), 6.36(s, 1H, C 6 h 4 ), 6.95(s, 1H, C 6 h 4 ), 7.37 (d, J=7.9Hz, 1H, C 6 h 4 );
[0024] 13 C NMR (150MHz, CDCl 3 )δ: 25.8(C-4'), 28.9(C-4), 37.9(C-a), 40.3(C-a'), 42.3(N-Me), 43.9(N-Me), 45.3(C-3') , 51.2(C-3), 55.0(OMe), 56.0(OMe), 61.9(C-1'), 64.2(C-1), 100.4(O-CH 2 -O), 102.2(C-5′), 110.9(C-13), 111.1(C-5), 116.8(C-10), 118.5(C-8), 120.9(C-11′), 122.2( C-13'), 12...
Embodiment 3
[0040] Determination of Antitumor Activity of (1R,1′R)-Stephalin
[0041] 1. Principle of antitumor activity
[0042] MTT method is a method to detect cell survival and growth. Based on the living cell metabolite reducing agent thiazolyl blue [3-(4,5-dimethyl-2-thiazole)-2,5-diphenyltetrazolium bromide, MTT]. MTT is a dye that accepts hydrogen atoms. The NADP-related dehydrogenase in the mitochondria of living cells can convert yellow MTT into insoluble blue-purple formazon (formazon), while dead cells have no such function. After dissolving formazon with DMSO, measure the optical density value with a microplate reader at a certain wavelength, which can quantitatively measure the survival rate of cells. The inhibitory effect of the sample on the tumor cells was observed according to the change of the optical density value.
[0043] 2. Antitumor activity experiment
[0044] Cell lines: human cervical cancer cells (Hela), human lung cancer cells (A549), human breast cancer ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com