Fusion protein
A technology of fusion protein and binding protein, applied in the field of fusion protein, can solve the difficult problems of phosphatidylserine eversion tracking and drug targeting
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0040] Example 1 Membrane Expression Functional Annexin A5 Fusion Protein Construction and Expression
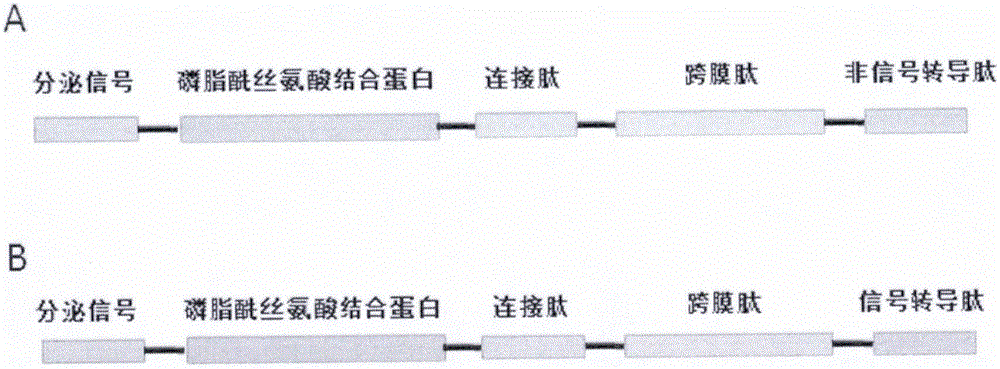
[0041] This embodiment provides a fusion protein, its construction method is as follows figure 1 As shown in B, it includes the first peptide segment and the second peptide segment. The first peptide segment includes the secretion signal peptide and the phosphatidylserine binding protein peptide segment, and the second peptide segment segment includes the connecting peptide segment and the transmembrane peptide segment. , and signal transduction peptides with cell regulation functions. The specific experimental steps are as follows:
[0042] 1. Construction of the second peptide region of the fusion protein
[0043] The commercial eukaryotic expression plasmid pEGFP-N1 (SEQ ID NO: 1) was selected as the vector. Gene synthesis encodes the nucleotide sequence of the second peptide region of the fusion protein (SEQ ID NO: 2), which sequence includes the Hind III site, the Ko...
Embodiment 2
[0051] Example 2 Membrane expression of non-functional annexin A5 fusion protein construction and expression
[0052] This embodiment provides a fusion protein, its construction method is as follows figure 1 As shown in A, it includes the first peptide region and the second peptide region. The first peptide region includes the secretion signal peptide and the phosphatidylserine binding protein peptide, and the second peptide region includes the connecting peptide and the transmembrane peptide. part. The specific experimental steps are as follows:
[0053] 1. Construction of the second peptide region of the fusion protein
[0054] Gene synthesis encodes the nucleotide sequence of the second peptide region of the fusion protein (SEQ ID NO: 9), which sequence includes Hind III site, Kozak sequence gccacc, translation initiation codon ATG, and 3xGGGGS peptide chain coding sequence from the 5' end Nucleic acid sequence, CD8a fragment (382nt-629nt in P01732-1), BamHI site.
[00...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com