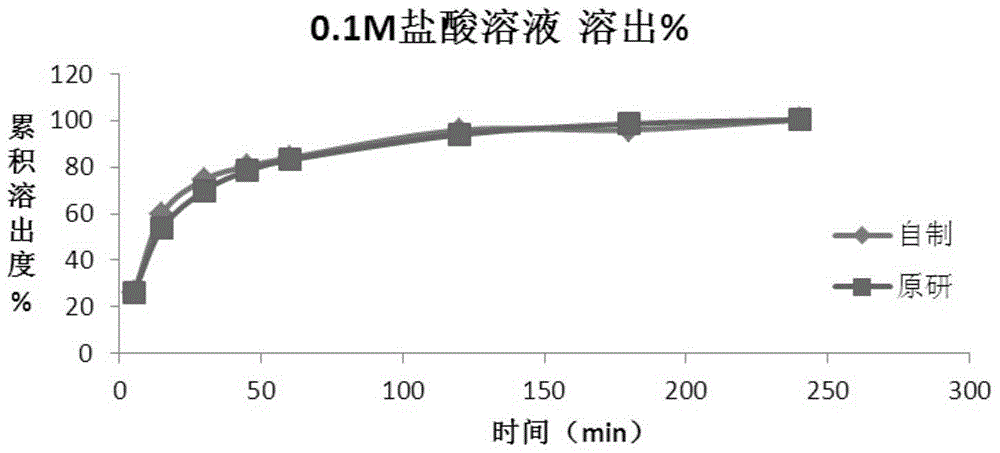
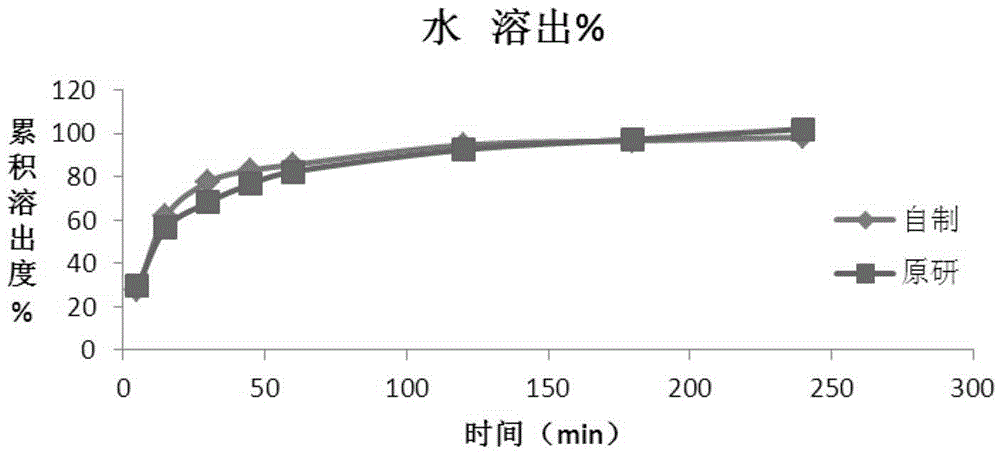
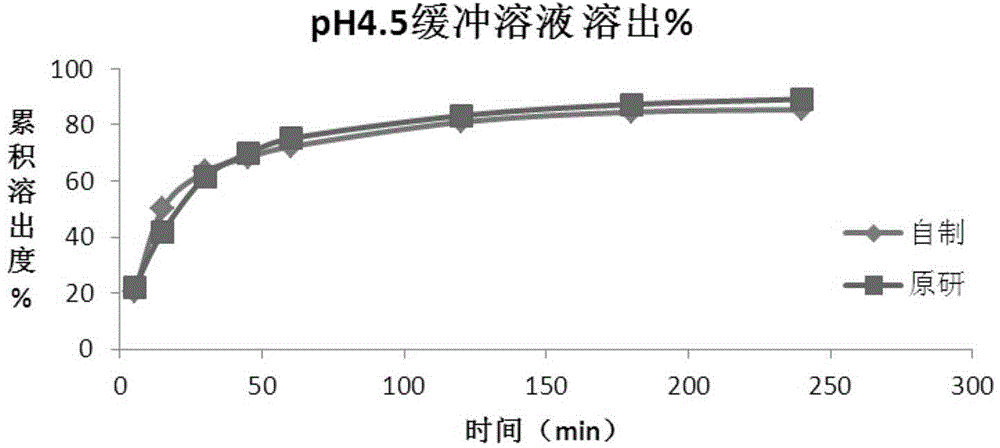
Method for preparing roflumilast tablets
A technology for roflumilast tablets and tableting, which is applied in the field of preparing roflumilast tablets, can solve problems such as being unfavorable to large-scale production, cumbersome process, easy consumption and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0032] The selection test of embodiment 1 principal agent dissolving solvent
[0033] Take about 0.5 g of the raw materials and put them in a beaker, gradually add ethanol solutions of different concentrations (50%, 75%, 95%), observe while shaking until the solution is clear, and weigh the amount of added solvent. According to the amount of different ethanol concentrations, 250g of mixed auxiliary materials were used to make soft materials, and the humidity of the soft materials was observed, and sieved with a 20-mesh sieve. The results are shown in Table 1.
[0034] Table 1 The selection and investigation results of the main ingredient dissolving solvent
[0035]
[0036] Result: The above-mentioned ethanol solutions with different concentrations can dissolve 500mg of raw materials (1000 prescriptions) well under a certain amount, and the amount of solvent has a good wetting effect on 1000 prescriptions (about 250g) To determine the formability of this product, use 50-95...
Embodiment 2
[0037] Embodiment 2 The screening test of roflumilast tablet binder of the present invention
[0038] Because this product is a small-scale drug, the proportion of the raw material in the preparation is very small, and the content uniformity is one of the main quality control indicators. In order to ensure the content uniformity, the raw and auxiliary materials need to be directly mixed with the raw material. The process is complex and difficult to control Therefore, it is planned to uniformly disperse the raw material drug in the solvent for granulation, and select different types of binders such as hydroxypropyl cellulose (HPC), hypromellose (HPMC), povidone K30 (PVPK30) , povidone K90 (PVPK90), carboxymethyl cellulose sodium (CMC-Na) and starch, were prepared with water and 50% ethanol respectively into the binder solution of conventional concentration, then added the same amount (0.5g) of the main drug , Observe the dissolution situation and phenomenon. The results are sh...
Embodiment 3
[0044] Embodiment 3 The proportioning test of principal ingredient and binder in the roflumilast tablet of the present invention
[0045] The raw materials and PVPK90 were added to appropriate amount of 50% ethanol in different proportions, stirred to disperse and dissolve, and the dispersion and dissolution conditions were observed.
[0046] Table 4 Dissolution investigation results of different ratios of main drug and PVPK90
[0047]
[0048] Results: When the ratio of raw material to PVPK90 is 1:3 or above, the raw material drug can be completely dissolved into a solution, and the ratio of API to PVPk90 in the prescription of this product is determined to be 1:3 or above.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Hardness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com