Preparation method of clarithromycin dispersible tablet
A technology for clarithromycin and dispersible tablets, which is applied in the field of preparation of clarithromycin dispersible tablets, can solve the problems of increasing process operation, high input cost, easy loss of raw materials, etc., and achieves reduction of process operation steps, reduction of input cost and savings energy effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0025] The selection test of embodiment 1 clarithromycin dissolving solvent
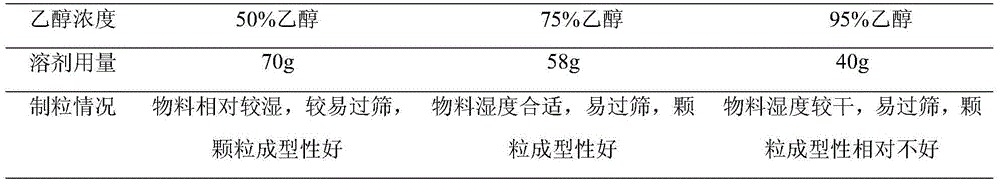
[0026] Take about 200 mg of the raw materials and put them in a beaker, gradually add ethanol solutions of different concentrations (50%, 75%, 95%), observe while shaking until the solution is clear, and weigh the amount of solvent added. According to the dosage of different ethanol concentrations, 800 mg of mixed auxiliary materials were used to make soft materials, and the humidity of the soft materials was observed, and sieved with a 20-mesh sieve. The results are shown in Table 1.
[0027] Table 1 The selection and investigation results of the main drug dissolution solvent
[0028]
[0029] Results: The above-mentioned ethanol solutions with different concentrations can dissolve 200 mg of raw materials well under a certain amount, and the amount of solvent has a good wetting effect on 800 mg of auxiliary materials, and it has good formability on material particles. It is determined that the gr...
experiment example 2
[0031] The prescription of clarithromycin tablets consists of the ingredients shown in Table 2.
[0032] Table 2 Prescription of clarithromycin tablets
[0033]
[0034]
[0035] The preparation method of above-mentioned clarithromycin dispersible tablet is as follows:
[0036] Preparation Process:
[0037] 1. Dissolve the prescribed amount of clarithromycin and hydroxypropyl methylcellulose in about 50g of 75% ethanol solution at the same time, and use it as an adhesive containing the main drug. The adhesive is colorless and transparent without lumps Blocks and bubbles.
[0038] 2. Take the prescribed amount of lactose, corn starch, cross-linked polyvinylpyrrolidone, and sodium saccharin and mix them through a 100-mesh sieve for 3 times.
[0039] 3. Add the binder containing the main ingredient to the mixture in step 2, prepare the soft material, granulate with a 20-mesh sieve, and dry in an oven at 60°C (moisture content 1-3%).
[0040] 4. The granules are passed t...
experiment example 3
[0043] The prescription of clarithromycin tablet consists of the ingredients shown in Table 3.
[0044] Table 3 Prescription of clarithromycin tablets
[0045]
[0046] The preparation method of above-mentioned clarithromycin dispersible tablet is as follows:
[0047] Preparation Process:
[0048] 1. Dissolve prescription amount of clarithromycin and hydroxypropyl cellulose ultrasonically in about 70g of 50% ethanol solution at the same time, as a binder containing the main ingredient, the binder is colorless and transparent, without agglomerates and bubble.
[0049] 2. Take the prescribed amount of lactose, corn starch, croscarmellose sodium, and aspartame and mix them through a 100-mesh sieve for 3 times.
[0050] 3. Add the binder containing the main ingredient to the mixture in step 2, prepare the soft material and granulate it with a 20-mesh sieve, and dry it in an oven at 40°C (moisture content 1-3%).
[0051] 4. The granules are passed through a 24-mesh sieve for...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com