Process for preparing concentrated sulfuric acid from industrial sulfur-containing tail gas
A concentrated sulfuric acid and tail gas technology, which is applied in the chemical industry, sulfur compounds, climate sustainability, etc., can solve the problems of large equipment investment and high operating cost, and achieve the effect of low equipment investment, relatively low operating cost, and low operating cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0023] In this example, the sulfur-containing waste gas data discharged by a certain device is used. It is determined that the gas volume of the tail gas is 2000 kmol / h, the temperature is 380 ° C, the pressure is 260 kPaG, and the molar composition is: N 2 84.9%, CO 2 10.6%, O 2 2.5%, SO 2 1.0%, H 2 O 1.0%.
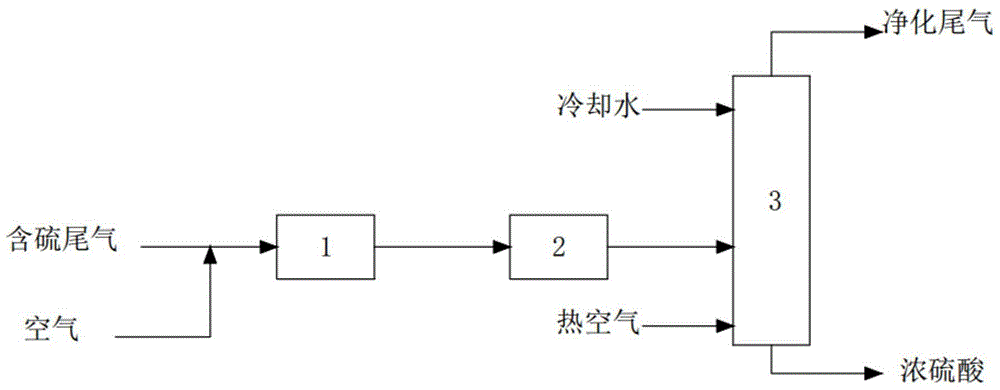
[0024] like figure 1 As shown, the sulfur-containing tail gas and air enter the conversion reactor 1, the outlet temperature is controlled at 410°C, the operating pressure is controlled at 255kPaG, the catalyst is a vanadium-based catalyst, SO 2 The conversion rate was 99.7%. Afterwards, heat is recovered by the waste heat recovery system 2 and cooled to 280°C (about 30°C higher than the dew point of sulfuric acid), and enters the acid tower 3, and 40°C cooling water is added to the top of the tower, and the gas phase is partially condensed into acid. The operation of the acid tower 3 The temperature is controlled at 70-240°C, the operating pressure is controlled a...
Embodiment 2
[0027] In this example, the sulfur-containing waste gas data discharged by a certain device is used. It is determined that the gas volume of the tail gas is 2000 kmol / h, the temperature is 420 ° C, the pressure is 110 kPaG, and the molar composition is: N 2 82.2%, CO 2 10.3%, O 2 2.5%, SO 2 1.0%, H 2 O 4.0%.
[0028] like figure 1 As shown, the sulfur-containing tail gas and air enter the conversion reactor 1, the outlet temperature is controlled at 450°C, the operating pressure is controlled at 105kPaG, the catalyst is an iron-based catalyst, SO 2 The conversion rate was 99.5%. Afterwards, the heat is recovered by the waste heat recovery system 2 and cooled to 280°C (about 22°C higher than the dew point of sulfuric acid), and then enters the acid tower 3, adding cooling water at 40°C to the top of the tower, and stripping with dry air at 100°C at the bottom of the tower. Partially condensed into acid, the operating temperature of the acid tower 3 is controlled at 70-...
Embodiment 3
[0031] In this example, the sulfur-containing waste gas data discharged by a certain device is used. It is determined that the gas volume of the tail gas is 2000 kmol / h, the temperature is 400 ° C, the pressure is 10 kPaG, and the molar composition is: N 2 76.9%, CO2 9.6%, O 2 2.5%, SO 2 1.0%, H 2 O 10.0%.
[0032] like figure 1 As shown, the sulfur-containing tail gas and air enter the conversion reactor 1, the outlet temperature is controlled at 430°C, the operating pressure is controlled at 8kPaG, and the catalyst is a noble metal catalyst, SO 2 The conversion rate was 99.6%. After that, the heat is recovered by the waste heat recovery system 2 and cooled to 280°C (about 25°C higher than the dew point of sulfuric acid), and then enters the acid tower 3, adding cooling water at 40°C to the top of the tower, and stripping with dry air at 100°C at the bottom of the tower. Partially condensed into acid, the operating temperature of the acid tower 3 is controlled at 60-...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com