Chimeric antigen receptor and cell expressing same and preparation method and application of chimeric antigen receptor
A chimeric antigen receptor and cell technology, applied in the field of tumor cell immunotherapy, can solve the problems of poor treatment effect, inability to perform surgical resection, low survival rate, etc., and achieve good killing effect.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0038] Embodiment 1 CAR plasmid construction
[0039] 1. Plasmid construction
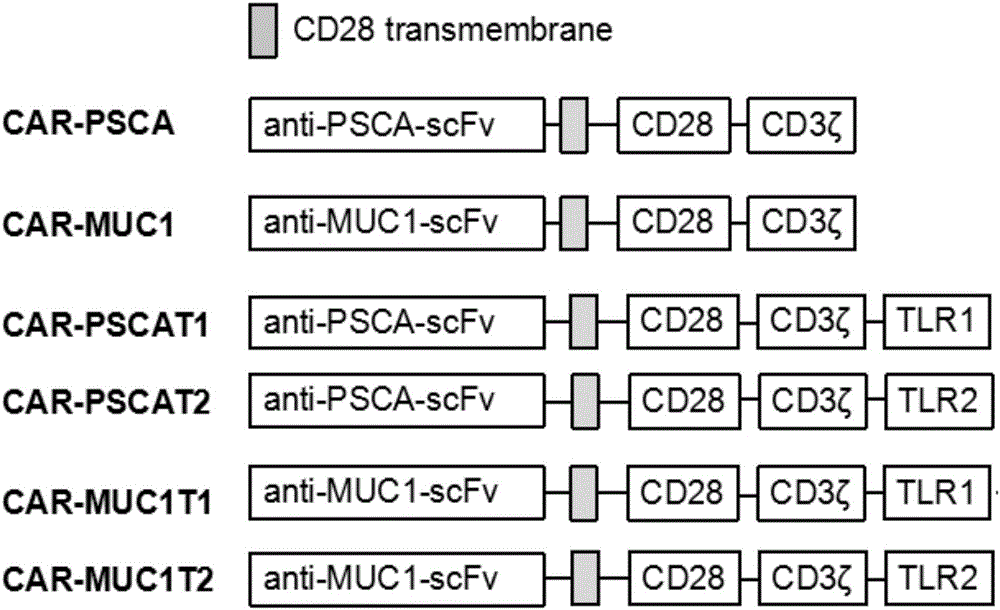
[0040] 1) Through gene synthesis, respectively synthesize CAR-PSCA, CAR-MUC1, CAR-PSCA-T1, CAR-PSCA-T2, CAR-MUC1-T1, and CAR-MUC1-T2 (gene sequence map as shown in figure 1 shown), the C-terminal of the synthesized gene contains a restriction endonuclease Pme1 restriction site and its protective bases, and the N-terminal contains a restriction endonuclease Spe1 restriction site and its protective bases.
[0041] For the synthetic gene sequence see figure 1 Sequence map, according to SEQ NO.1 (for the DNA sequence of Anti-PSCA-scFV) and SEQ NO.2 (for the DNA sequence of Anti-MUC-1-scFV) and SEQ NO.3 (for the DNA sequence of the TLR1 domain ) and SEQ NO.4 (the DNA sequence of the TLR2 domain), SEQ NO.5 (the DNA sequence of the CD3ζ domain) and SEQ NO.6 (the DNA sequence of the CD28 intracellular domain).
[0042] 2) Obtain synthetic DNA fragments (CAR-PSCA, CAR-MUC1, CAR-PSCA-T1, CAR-PSCA-T2, CAR-...
Embodiment 2
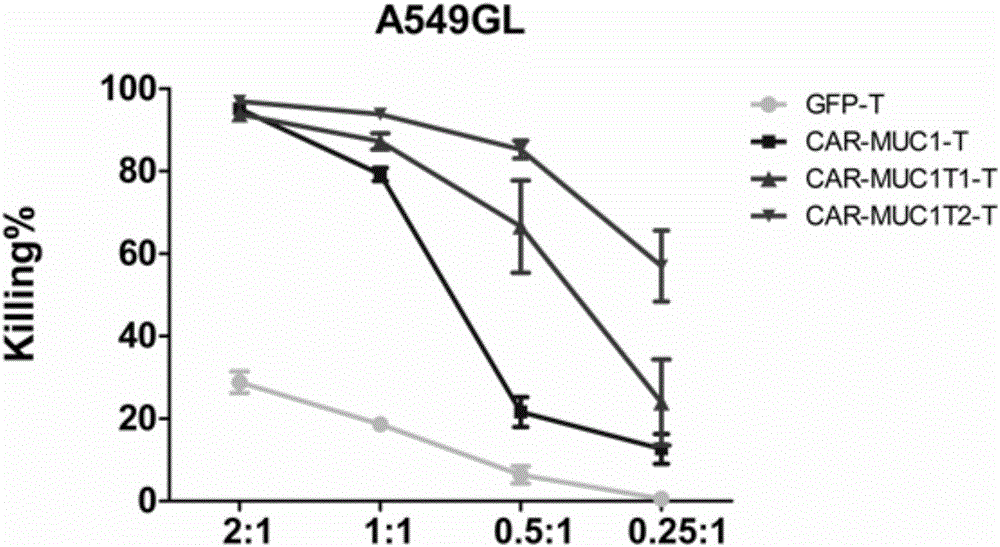
[0061] Example 2 In vitro detection of the killing function of CAR-MUC1-T1 / 2T cells on tumor (lung cancer) cells
[0062] 1) The GFP T (blank control), CAR-MUC1T (negative control), CAR-MUC1-T1T, and CAR-MUC1-T2T cells prepared in Example 1 were mixed with 1×10 4 The tumor cells A549-GL were mixed at the ratio of 2:1, 1:1, 0.5:1, and 0.25:1, and added to 96-well U-shaped plate, with 3 duplicate holes for each group, centrifuged at 250g for 5min, and placed at 37 ℃5%CO 2 Co-culture in the incubator for 18 hours;
[0063] 2) The recognition and killing functions of GFP T, CAR-MUC1T, CAR-MUC1-T1T, and CAR-MUC1-T2T cells on lung cancer cells were compared in vitro. A549-GL human lung adenocarcinoma cell line with luciferase was used as tumor cells.
[0064] 3) Luciferase (Luciferase) quantitative killing efficiency evaluation method: 18 hours after CAR T cells were co-cultured with tumor cells (the experimental control group was cultured with tumor cells alone), 100 μl / well of l...
Embodiment 3
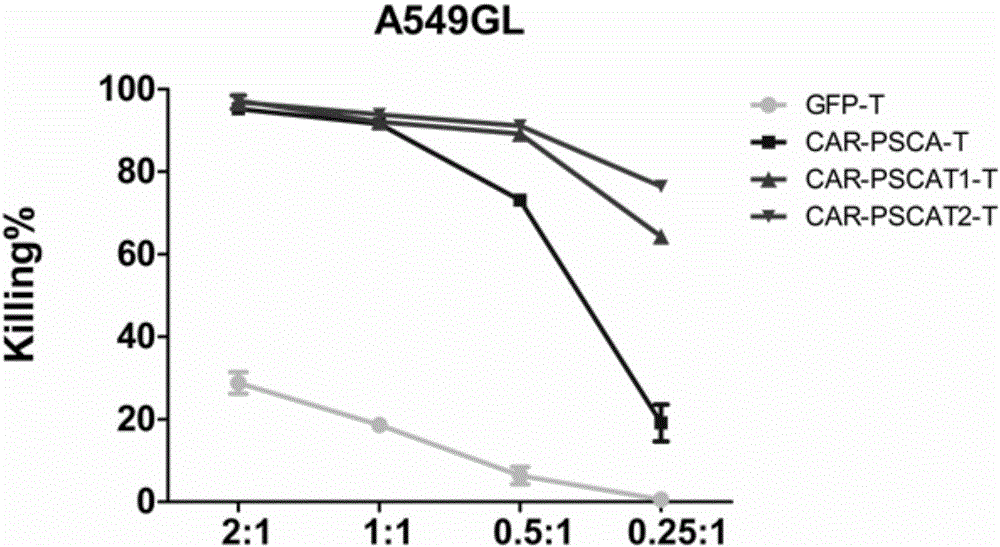
[0066] Example 3 In vitro detection of the killing function of CAR-PSCA-T1 / 2T cells on tumor (lung cancer) cells
[0067]1) The GFP T (blank control), CAR-PSCA T (negative control), CAR-PSCA-T1T, and CAR-PSCA-T2T cells prepared in Example 1 were mixed with 1×10 4 The tumor cells A549-GL were mixed at the ratio of 2:1, 1:1, 0.5:1, and 0.25:1, and added to 96-well U-shaped plate, with 3 duplicate holes for each group, centrifuged at 250g for 5min, and placed at 37 ℃5%CO 2 Co-culture in the incubator for 18 hours;
[0068] 2) The recognition and killing functions of GFP T, CAR-PSCA T, CAR-PSCA-T1T, and CAR-PSCA-T2T cells on lung cancer cells were compared in vitro, and the human lung adenocarcinoma cell line A549-GL with luciferase was selected as the tumor cells.
[0069] 3) Luciferase (Luciferase) quantitative killing efficiency evaluation method: 18 hours after CAR T cells were co-cultured with tumor cells (the experimental control group was cultured with tumor cells alone),...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com