Chicken inclusion body hepatitis inactivated vaccine and preparation method thereof
An inactivated vaccine and inclusion body technology, which is applied to the field of chicken inclusion body hepatitis inactivated vaccine and its preparation, can solve the problem that chicken inclusion body hepatitis cannot be prevented by vaccines, etc., and reduce the infection rate of bacterial diseases such as Escherichia coli and increase the The immune function of the body, the effect of strong disease resistance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0028] 1. Antigen preparation
[0029] 1.1 Inoculation Dilute the seed poison 15,000 times with sterile saline, inoculate 7-day-old SPF chicken embryos through the yolk sac, 0.1ml per embryo, seal the holes with wax, and incubate at 37°C.
[0030] 1.2 Incubation and observation Discard the chicken embryos that died 48 hours ago, and light the eggs every 6 hours thereafter, collect the chicken embryos that died within 48-120 hours, and refrigerate them at 2-8°C for 12-24 hours.
[0031] 1.3 Harvest Chicken embryos were disinfected with tincture of iodine to disinfect the air cells on the surface of the eggshells, remove the eggshells from the air cells by aseptic operation, and harvest allantoic fluid and embryo bodies. Among them, the allantoic fluid is placed in a sterilized 500ml glass bottle with a capacity of 450ml / bottle, labeled as chicken inclusion body hepatitis virus SDJ15-8 strain E8, and stored at -20°C or below; the embryo body is mashed with tissue Machine crushe...
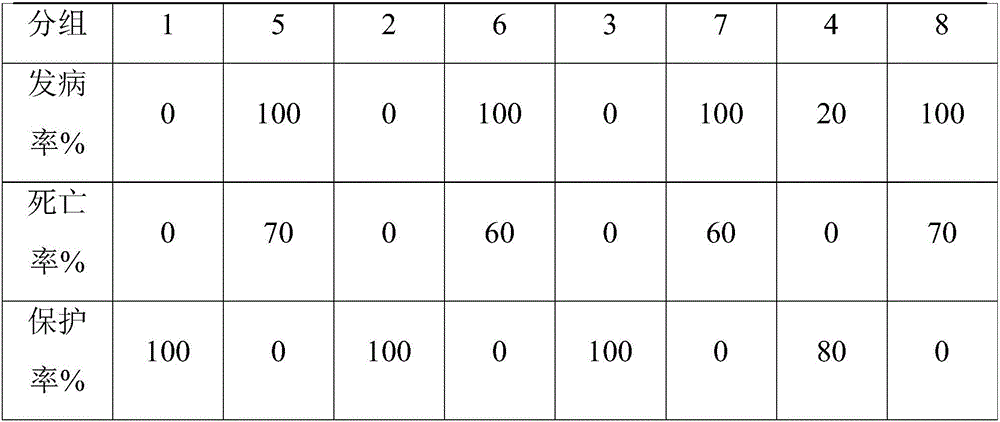
Embodiment 2
[0058] 1. Experiment on 5-day-old chicks
[0059] 1.1 Method
[0060] 140 5-day-old AA broiler chickens were divided into 7 groups, 20 in each group. Groups 1-6 were injected intramuscularly with chicken inclusion body hepatitis transfer factor adjuvant inactivated vaccine 1-6, 0.3ml / bird. Group 7 was The control group received no treatment. Fifteen days after immunization, intramuscularly inoculate chicken inclusion body hepatitis virus SDJ15-8 strain 100LD50 per chick in groups 1-7, observe for 10 days after virus inoculation, and count the morbidity and mortality of chicks in each group.
[0061] 1.2 Results
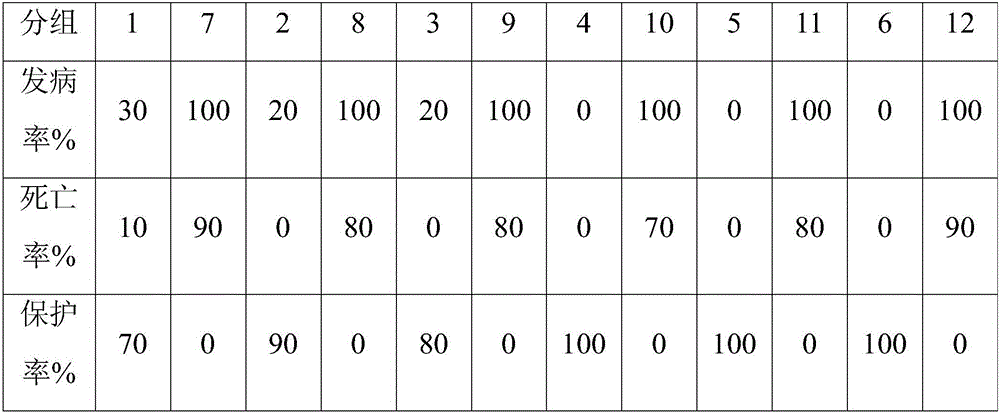
[0062] When the virus content of the antigen before inactivation of the vaccine is ≥10-6.0ELD50 / 0.1ml, and the polypeptide content is 2mg / ml, the chicks can be 100% protected. The specific results are shown in Table 1.
[0063] Table 1 The incidence rate and protection rate of chicks in each group
[0064] group
1
2
3
4
5
6
7
I...
Embodiment 3
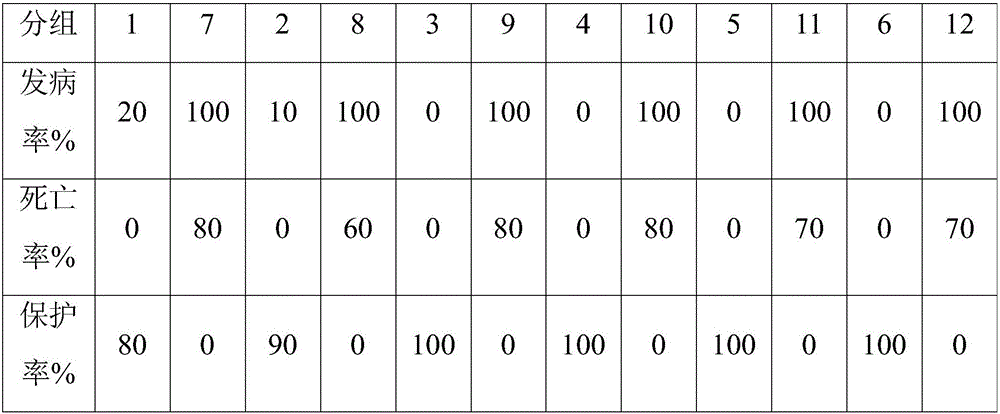
[0077] Experiment of the earliest time to produce protective antibody after immunizing chickens of different ages with chicken inclusion body hepatitis virus inactivated vaccine
[0078] 15-day-old chick test
[0079] 1.1 Method
[0080] 120 5-day-old AA broiler chicks were divided into 12 groups with 10 birds in each group. Groups 1-6 were immunized with chicken inclusion body hepatitis virus serum type 4 transfer factor adjuvant inactivated vaccine 0.3ml / piece by intramuscular injection, 7- Group 12 served as the control group without any treatment. At 8, 9, 10, 11, 12, and 13 days after immunization, treat all chicks in groups 1 and 7, groups 2 and 8, groups 3 and 9, groups 4 and 10, groups 5 and 11, groups 6 and 12, respectively Intramuscular Inoculation of Chicken Inclusion Body Hepatitis Virus SDJ15-8 Strain 100LD 50 / Only. The above-mentioned groups were observed for 10 days after being inoculated with the virus, and the morbidity and mortality of chicks in each gro...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com