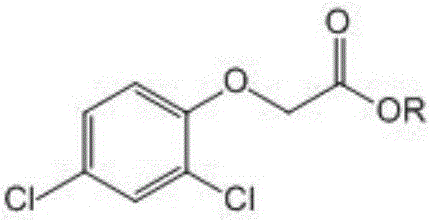
Chloracetate water phase synthesis technology and novel method for preparing 2,4-D ester
A technology of chloroacetate and chloroacetic acid, applied in the field of preparation of 2,4-D ester, can solve the problems of high cost, long reaction time, complicated operation, etc., and achieves avoiding a large amount of use and waste, simple and convenient post-treatment process, good purity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0040] Example 1 Aqueous phase synthesis technology of methyl chloroacetate and preparation of 2,4-D methyl ester
[0041] Add 205g of methanol (6.4mol), 771.4g of chloroacetic acid (8mol) and 400g of water into a 2000mL three-neck flask equipped with a thermometer, a stirring device and a reflux device, mix and stir and heat up to 90°C, react for 6h, and stir and heat As well as the esterification reaction, the azeotropic system formed by methanol, methyl chloroacetate and water first passes through a reboiler with hot water at 78°C, and the methanol solution with a low boiling point passes through the reboiler and flows upward through condensation. In the reaction bottle, the high-boiling methyl chloroacetate solution is condensed and enters the water separator for stratification, the lower layer of methyl chloroacetate is released and collected, and the upper layer aqueous solution is pumped back into the reaction bottle.
[0042] Mix and stir 83.2g (0.5mol) of 2,4-dichloro...
Embodiment 2
[0043] Example 2 Aqueous Phase Synthesis Technology of Butyl Chloroacetate and Preparation of 2,4-D Butyl Ester
[0044] Add 185g of butanol (2.5mol), 241.1g of chloroacetic acid (2.5mol) and 300g of water into a 3000mL three-necked flask equipped with a thermometer, a stirring device and a reflux device, mix and stir and heat up to 120°C, react for 8 hours, and heat with stirring As well as the esterification reaction, the azeotropic system formed by butanol, butyl chloroacetate and water first passes through a reboiler with hot water at 125°C, and after the butanol and aqueous solution pass through the reboiler, they are condensed and flow back upwards. In the reaction bottle, the butyl chloroacetate with high boiling point is condensed and enters the water separator for stratification, the butyl chloroacetate in the lower layer is released and collected, and the azeotropic solution of the upper layer aqueous solution is pumped back into the reaction bottle.
[0045] 83.2g (...
Embodiment 3
[0046] Example 3 Aqueous Phase Synthesis Technology of Isooctyl Chloroacetate and Preparation of 2,4-D Isooctyl
[0047] Add 390g of isooctyl alcohol (3mol), 212.1g of chloroacetic acid (2.2mol) and 500g of water into a 3000mL three-necked flask equipped with a thermometer, a stirring device and a reflux device, mix and stir and raise the temperature to 150°C, and react for 7 hours. Heating and esterification reaction, the azeotropic system formed by isooctyl alcohol, isooctyl chloroacetate and water first passes through a reboiler at 110°C, and the aqueous alcohol solution passes through the reboiler, then flows upward through condensation and returns to the reaction bottle , the high-boiling isooctyl chloroacetate solution is condensed into the water separator for stratification, the lower layer of isooctyl chloroacetate is released and collected, and the upper layer aqueous solution is pumped back into the reaction bottle.
[0048] 83.2g (0.5mol) of 2,4-dichlorophenol, 300g...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com