Fosfluconazole freeze-drying preparation composition and preparing method thereof
A technology of fosfluconazole and freeze-dried preparations, applied in freeze-dried transportation, antifungal agents, pharmaceutical formulations, etc., can solve the problems of unfavorable promotion and application, increased cost, inconvenient storage, etc., and achieve strong resolubility and good stability performance, the effect of improving practicality
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
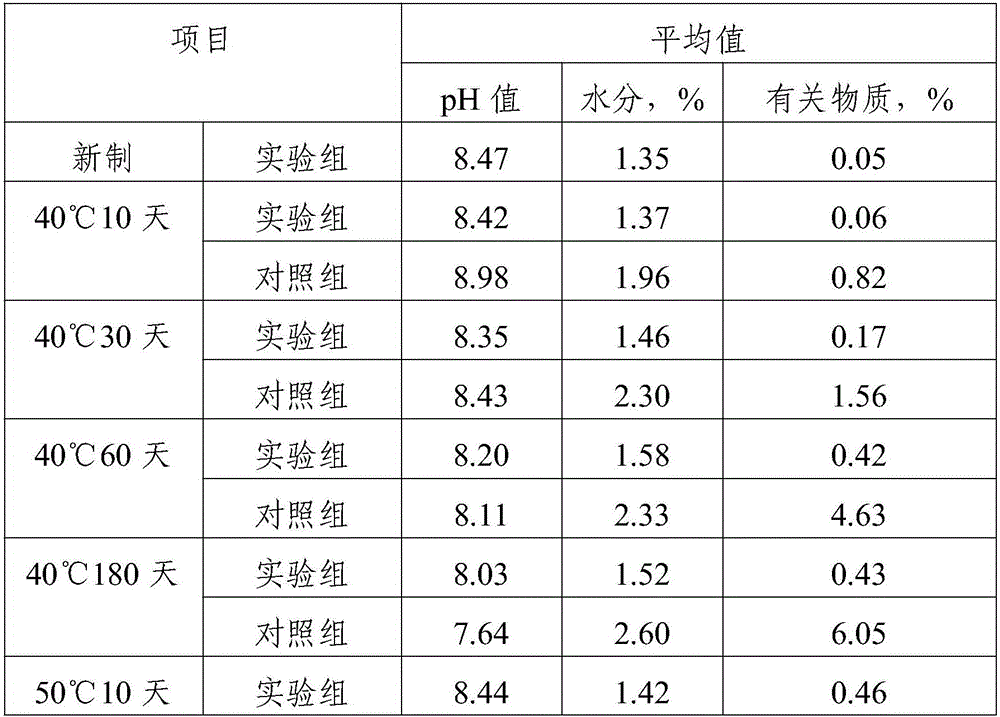
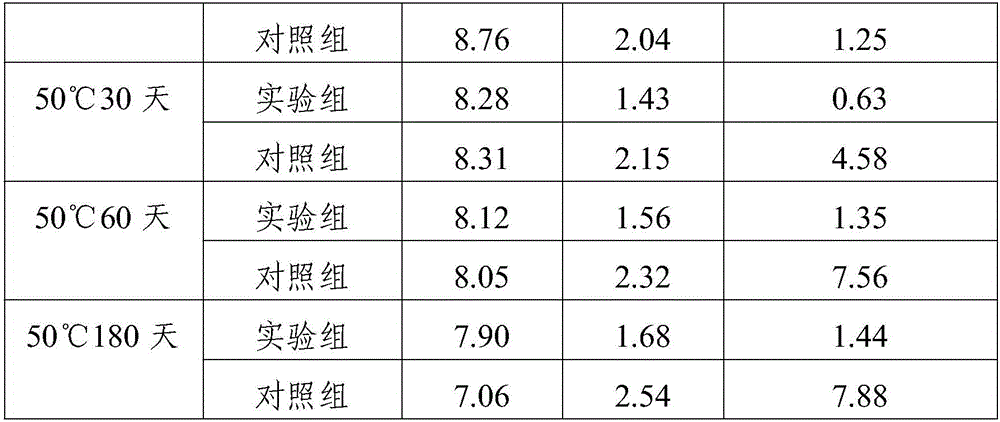
[0018] A kind of fosfluconazole freeze-dried preparation composition, comprising fosfluconazole, molar ratio is the composition of absolute ethanol and acetone of 1:1, the composition of lactic acid and sodium hydroxide, povidone K30, glucose and Composition of mannitol, glycine.
[0019] The mass percentage of fosfluconazole and filler is 1:2.6.
[0020] The mass percentage of excipient, stabilizer and povidone K30 is 1.8:0.9:1.1.
[0021] The preparation method of fos fluconazole freeze-dried preparation composition, the preparation steps are as follows:
[0022] 1) Add fosfluconazole and the filler to water for injection 8 times the weight of the filler, stir and blend, add the composition of absolute ethanol and acetone dropwise during the stirring process, and then add 2 times the weight of the filler Water for injection in parts by weight, stirred evenly, and then adding a pH regulator to adjust the pH to 8.3 to obtain an aqueous solution of forsifluconazole;
[0023]...
Embodiment 2
[0026] A fosfluconazole freeze-dried preparation composition, comprising forsfluconazole, a composition of dehydrated alcohol and acetone with a molar ratio of 2:1, a composition of tartaric acid and sodium hydroxide, povidone K30, glucose, Composition of tryptophan and phenylalanine.
[0027] The mass percentage of fosfluconazole and filler is 1:2.5.
[0028] The mass percentage of excipient, stabilizer and povidone K30 is 2:0.8:1.2.
[0029] The preparation method of fos fluconazole freeze-dried preparation composition, the preparation steps are as follows:
[0030] 1) Add fosfluconazole and the filler to water for injection 8 times the weight of the filler, stir and blend, add the composition of absolute ethanol and acetone dropwise during the stirring process, and then add 2 times the weight of the filler Water for injection in parts by weight, stirred evenly and then adding a pH regulator to adjust the pH to 8.5 to obtain an aqueous solution of fluconazole fores;
[00...
Embodiment 3
[0034] A composition of fosfluconazole freeze-dried formulations, comprising the composition of fosfluconazole, molar ratio of absolute ethanol and acetone of 2.5:1, phosphate buffer, povidone K30, sorbitol and mannitol , aspartic acid.
[0035] The mass percentage of fosfluconazole and filler is 1:3.2.
[0036] The mass percentage of excipient, stabilizer and povidone K30 is 1.6:0.9:1.2.
[0037] The preparation method of fos fluconazole freeze-dried preparation composition, the preparation steps are as follows:
[0038] 1) Add fosfluconazole and the filler to water for injection 8 times the weight of the filler, stir and blend, add the composition of absolute ethanol and acetone dropwise during the stirring process, and then add 2 times the weight of the filler Water for injection in parts by weight, stirred evenly and then adding a pH regulator to adjust the pH to 8.2 to obtain an aqueous solution of fluconazole fores;
[0039] 2) filter and sterilize the fosfluconazole ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com