Chitosan oligosaccharide vaccine adjuvant based on chemical coupling and application of chitosan oligosaccharide vaccine adjuvant
A technology of vaccine adjuvant and chitosan oligosaccharide is applied in the field of biomedicine to achieve the effects of good adjuvant effect, enhanced immune response, and mature and stable preparation method.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
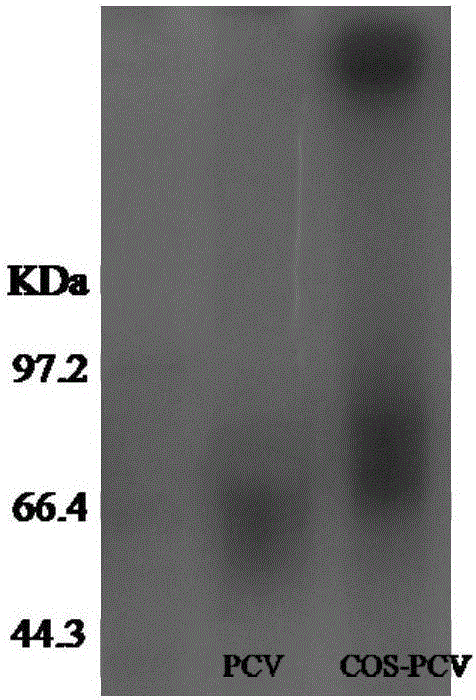
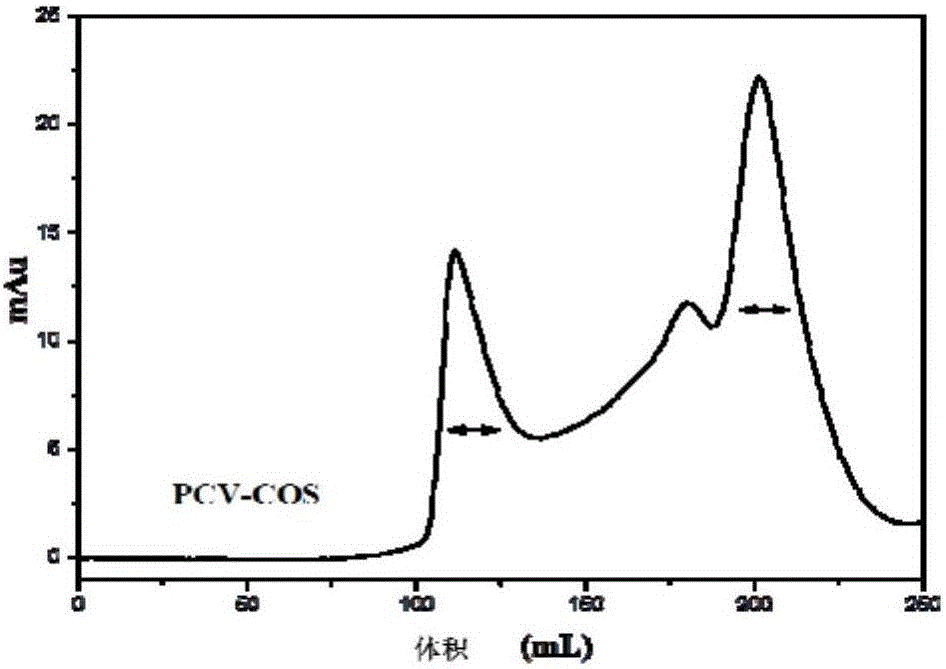
[0029] Embodiment 1: the preparation of chitosan oligosaccharide vaccine adjuvant
[0030] 1. Preparation of chitosan oligosaccharide adjuvant:
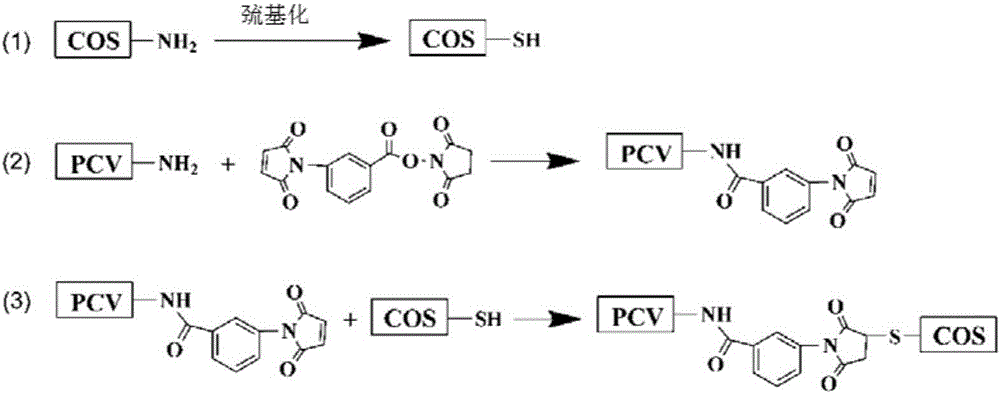
[0031] (1) Thiolylation derivatization of oligochitosan COS
[0032]Weigh the required mass of COS (mass ratio COS:PCV=1:1) and 2-iminosulfane hydrochloride (IT) respectively, take IT by the molar ratio of IT:COS=5:1, and use 20mM PBS (pH 7.4) was dissolved and mixed, and reacted at room temperature for 3 hours.
[0033] After the reaction time is up, use a G25 desalting column to elute to remove unreacted small molecules.
[0034] (2) Maleimidization Derivation of Porcine Circular Inactivated Vaccine PCV
[0035] Draw the PCV of 3mL1.7mg / mL according to the required amount, weigh 3-benzoylmaleimide-N-hydroxysuccinimide (MBS) by the molar ratio of MBS:PCV=100:1, First dissolve in DMF and then dissolve with 20mM PBS (pH 7.4), add PCV to mix, and react at 4°C for 3 hours, wherein the reaction buffer is 20mM PB (pH 7.2), and the vol...
Embodiment 2
[0041] Embodiment 2: the vaccine adjuvant activity determination of chitosan oligosaccharide vaccine adjuvant
[0042] The immune activity of the chitosan oligosaccharide conjugate in Example 1 was detected, the mice were immunized by intramuscular injection, and the antibody titer was determined. The specific method is as follows:
[0043] Experimental animals: C57BL / 6 mice, 4-6 weeks old, 6 mice / group, female.
[0044] Control solvent: phosphate buffer saline (PBS).
[0045] Dosage: porcine circular inactivated vaccine (PCV): 100 μg / mouse (mouse); chitooligosaccharide: 100 μg / mouse; chitooligosaccharide conjugate 1 (PCV-COS-1): 100 μg / mouse; shell Oligosaccharide conjugate 2 (PCV-COS-2): 100 μg / mouse; oil emulsion adjuvant (ISA206): 100 μg / mouse.
[0046] Grouping: (1) Blank control group (PBS); (2) Individual vaccine group (PCV): PBS+PCV; (3) Physical mixture group (PCV / COS): COS+PCV; (4) PCV-COS-1 Group: PBS+PCV-COS-1; (5) PCV-COS-2 group: PBS+PCV-COS-2; (6) oil emulsi...
Embodiment 3
[0060] Embodiment 3: Pathological section research of chitosan oligosaccharide vaccine adjuvant
[0061] The injection sites of each group of mice in Example 2 were collected, and then treated with conventional pathological methods. Specifically, the tissue at the injection site was fixed with 10% formalin solution, embedded in paraffin and cut into 4-mm sections, then stained with HE and observed and photographed with a microscope (Leica) under bright field.
[0062] Experimental results:
[0063] In order to verify whether there is possible adverse reaction in the chitosan oligosaccharide adjuvant of the present invention, collect the injection site of each immunization group mouse and carry out the comparison of pathological section ( Figure 5 ). The results showed that the oil-emulsion adjuvant group had an obvious inflammatory reaction at the injection site, but there was no significant change in the chitosan-oligosaccharide conjugate group and other groups. It shows ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| absorbance | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com