Cobalt-based catalyst for reverse water-gas shift
A technology of cobalt-based catalyst and reverse water gas, applied in the direction of physical/chemical process catalysts, chemical instruments and methods, chemical/physical processes, etc., can solve problems such as poor thermal stability and methanation side reactions, and achieve good stability and inhibition Effect of methanation reaction and inhibition of methanation side reaction
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
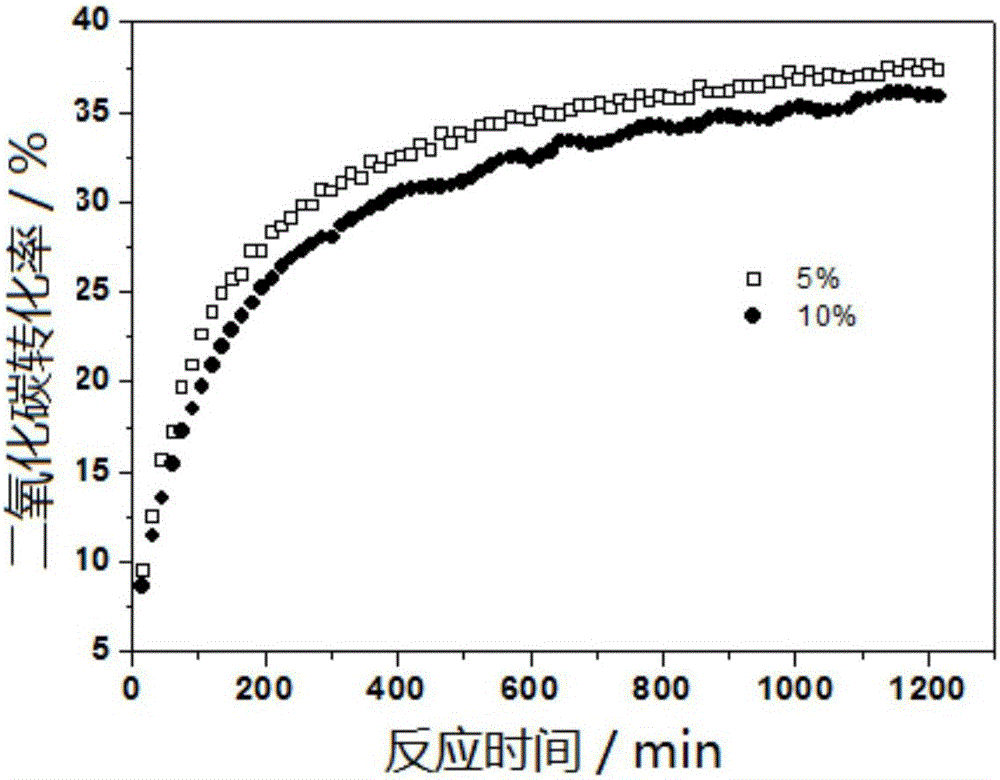
[0034] Taking the 5% Co / crab shell catalyst with a cobalt mass percentage of 5% as an example, it is prepared by an equal volume impregnation method, and the specific steps are as follows:
[0035] Clean the fresh Portunus trituberculatus shells, dry them at 100°C for 24 hours, place them in a muffle furnace and roast them at 600°C in an air atmosphere for 2 hours, cool them down to room temperature, and grind them to obtain the crab shell carrier. Weigh 0.2466g cobalt nitrate hexahydrate Co(NO 3 ) 2 ·6H 2 O was placed in a 50ml beaker, and 0.95ml deionized water was used to dissolve cobalt nitrate hexahydrate Co(NO 3 ) 2 ·6H 2 O gets Co(NO 3 ) 2 Solution, weigh 0.950g crab shell carrier with electronic balance balance, pour into Co(NO 3 ) 2 In the solution, it was ultrasonicated in an ultrasonic cleaner for 30 minutes, then placed in an oven at 30°C and 80°C for 24 hours, and after being completely dried, it was baked at 600°C in a muffle furnace for 2 hours to obtain...
Embodiment 2
[0037] Taking the 10% Co / crab shell catalyst with a cobalt mass percentage of 10% as an example, it is prepared by an equal volume impregnation method, and the specific steps are as follows:
[0038] Clean fresh portunus trituberculatus shells, dry them at 100°C for 24 hours, place them in a muffle furnace and roast them at 600°C in an air atmosphere for 3 hours, cool them down to room temperature, and grind them to obtain a crab shell carrier. Weigh 0.4932g cobalt nitrate hexahydrate Co(NO 3 )2 ·6H 2 O is placed in a 50ml beaker, and 0.90ml of deionized water is taken with a pipette gun to dissolve cobalt nitrate hexahydrate Co(NO 3 ) 2 ·6H 2 O gets Co(NO 3 ) 2 Solution, weigh 0.900g crab shell carrier with electronic balance balance, pour into Co(NO 3 ) 2 In the solution, sonicate in an ultrasonic cleaning machine for 30 minutes, then place in an oven at 30°C and 80°C for 24 hours, and after being completely dried, place it in a muffle furnace at 600°C for 2 hours to ...
Embodiment 3
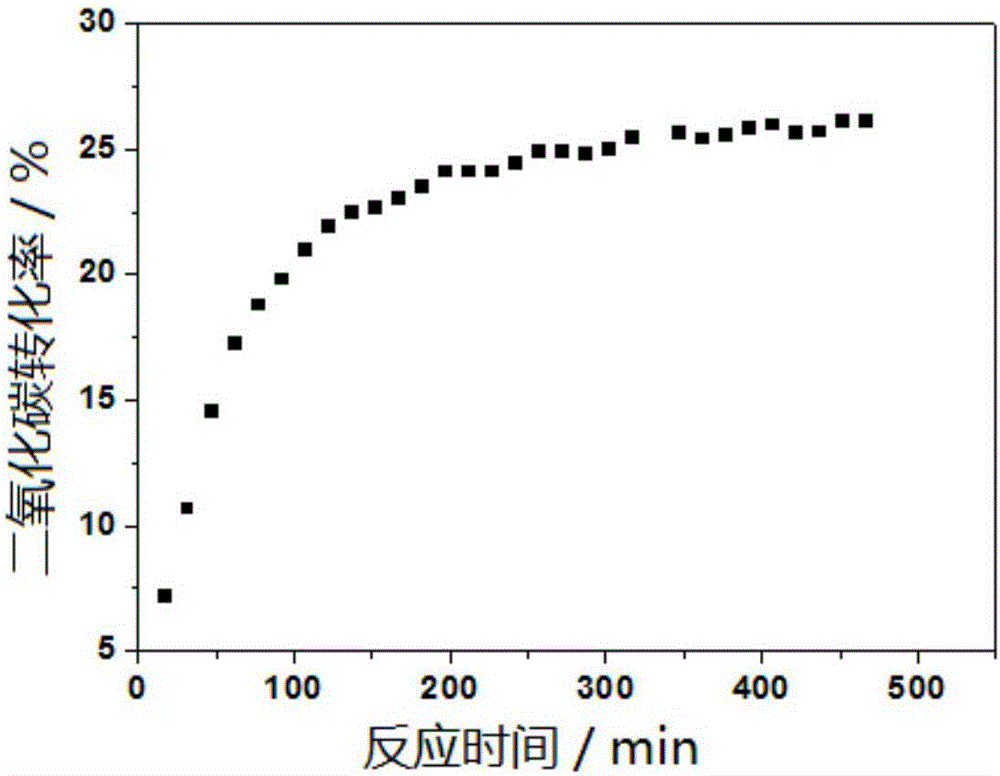
[0040] 600 2h-5% Co-CaCO with 5% cobalt mass percent 3 Catalyst is taken as an example, prepared by excessive impregnation method, the specific steps are as follows: CaCO 3 Placed in a muffle furnace at 600°C for 2 h and cooled to room temperature. Weigh 0.2466g cobalt nitrate hexahydrate Co(NO 3 ) 2 6H2O was placed in a 50ml beaker, and 10ml of deionized water was taken with a measuring cylinder to dissolve cobalt nitrate hexahydrate Co(NO 3 ) 2 ·6H 2 O gets Co(NO 3 ) 2 solution, weigh 0.950g CaCO with an electronic balance 3 , into Co(NO 3 ) 2 In the solution, place the beaker on a magnetic stirrer and stir for 2 hours, let it stand for stratification, extract the supernatant and discard it, and place it in an oven at 30°C and 80°C for 24 hours in turn, and after it is completely dry, place it in a muffle furnace at a temperature of 600°C 5% Co / CaCO was obtained after lower calcination for 2h 3 catalyst.
[0041] Take 0.0100g of the catalyst prepared in the above...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com