Halogenated-1-(4-mercaptobutyl)-3-methylimidazolium ionic liquid and preparation method and application thereof
A technology of methylimidazolium ion and mercaptobutyl group, which is applied in chemical instruments and methods, organic chemistry, instruments, etc., can solve the problems of weak fluorescence effect, long reaction cycle, complicated post-processing process, etc., and achieve repeatability and stability Good, short reaction cycle, and the effect of improving fluorescence sensitivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
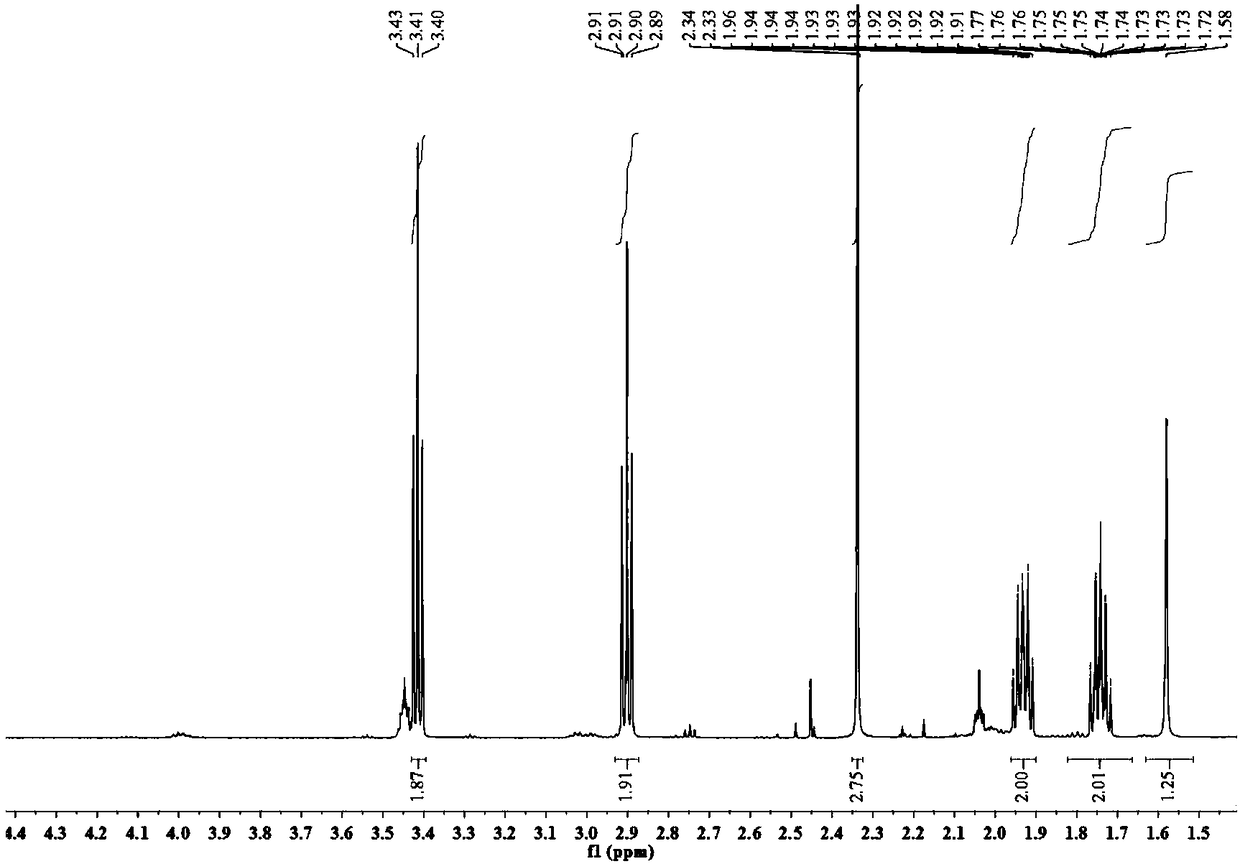
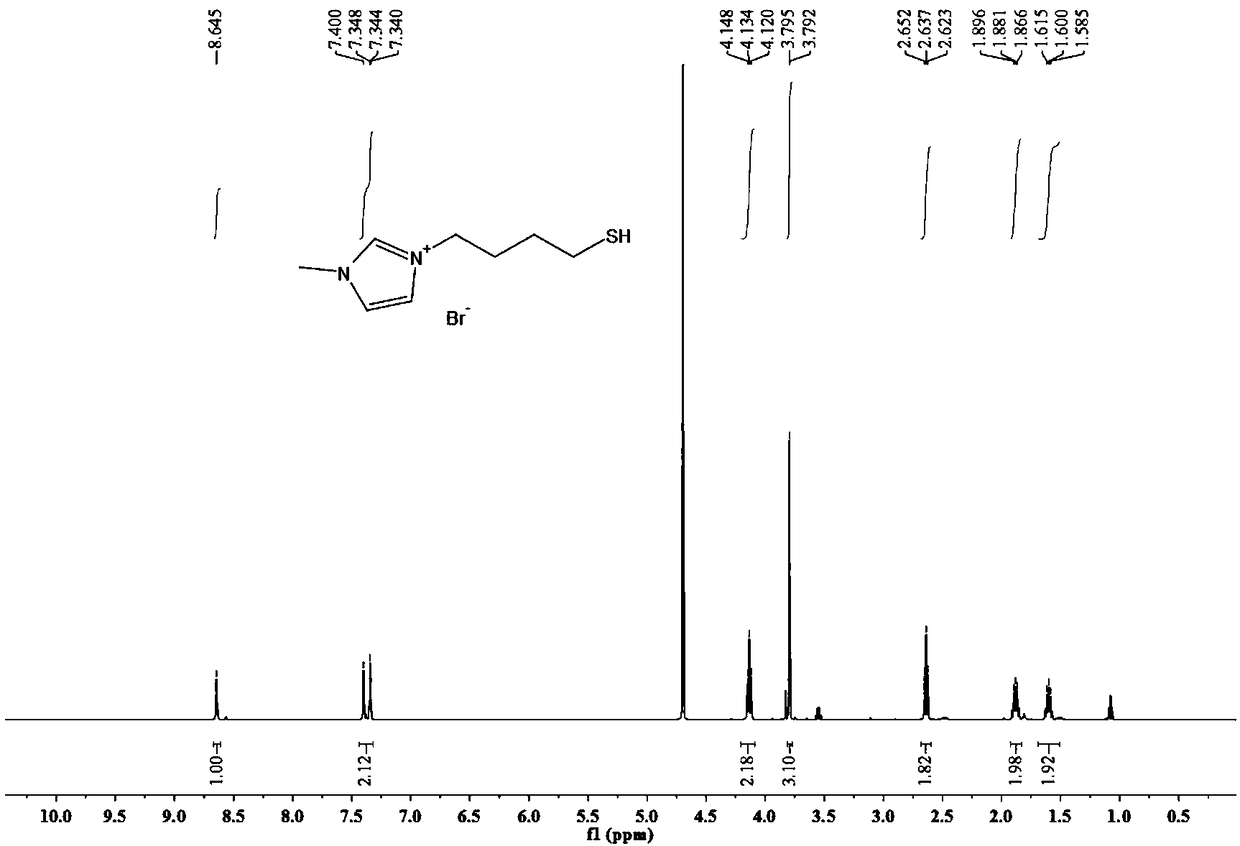
[0049] The bromide-1-(4-mercaptobutyl)-3-methylimidazolium ionic liquid of this embodiment has a structural formula as shown in formula II:
[0050]
[0051] The preparation method of bromide-1-(4-mercaptobutyl)-3-methylimidazolium ionic liquid of the present embodiment adopts the following steps:
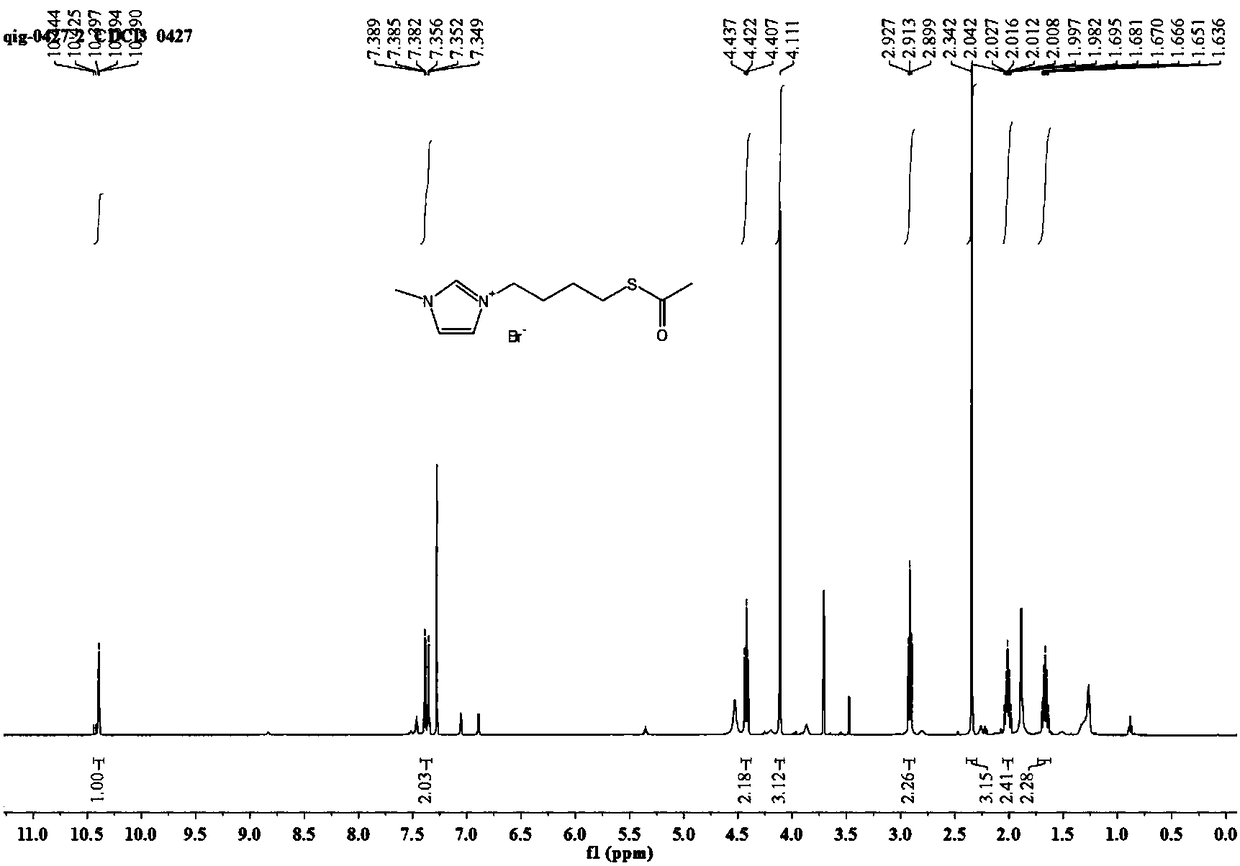
[0052] 1) Add 3.21g (15.0mmol) of 1,4-dibromobutane to a single-necked flask, add dichloromethane to dissolve, then weigh 2.05g (18.0mmol) of potassium thioacetate, add it to the system, and react at 35°C 8h, the system gradually turned yellow; the white solid was removed by suction filtration, the solvent was evaporated to dryness, and the mixed solvent of petroleum ether and dichloromethane with a volume ratio of 3:1 was used as the mobile phase for column chromatography separation to obtain 4-bromobutyl- Thioacetate 1.64g, yield rate is 52.1%;
[0053] 2) Weigh 0.52g (6.3mmol) of N-methylimidazole into a single-necked flask, add dichloromethane to dissolve, then add 1.2g (5....
Embodiment 2
[0056] The bromide-1-(4-mercaptobutyl)-3-methylimidazolium ionic liquid of this embodiment has the same structural formula as that of Embodiment 1.
[0057] The preparation method of bromide-1-(4-mercaptobutyl)-3-methylimidazolium ionic liquid of the present embodiment adopts the following steps:
[0058] 1) Add 3.05g (14.2mmol) of 1,4-dibromobutane to a single-necked flask, add dichloromethane to dissolve, then weigh 1.94g (17.0mmol) of potassium thioacetate and add it to the system, and react at 60°C 8h, the system gradually turned yellow; the white solid was removed by suction filtration, the solvent was evaporated to dryness, and the mixed solvent of petroleum ether and dichloromethane with a volume ratio of 3:1 was used as the mobile phase for column chromatography separation to obtain 4-bromobutyl- Thioacetate 1.01g, yield rate is 33.9%;
[0059] 2) Weigh 0.34g (4.2mmol) of N-methylimidazole into a single-necked flask, add dichloromethane to dissolve, then add 0.81g (3....
Embodiment 3
[0062] The bromide-1-(4-mercaptobutyl)-3-methylimidazolium ionic liquid of this embodiment has the same structural formula as that of Embodiment 1.
[0063] The preparation method of bromide-1-(4-mercaptobutyl)-3-methylimidazolium ionic liquid of the present embodiment adopts the following steps:
[0064] 1) Add 2.51g (11.7mmol) of 1,4-dibromobutane to a single-necked flask, add dichloromethane to dissolve, then weigh 1.60g (14.0mmol) of potassium thioacetate and add it to the system, and react at 10°C 8h, the system gradually turned yellow; the white solid was removed by suction filtration, the solvent was evaporated to dryness, and the mixed solvent of petroleum ether and dichloromethane with a volume ratio of 3:1 was used as the mobile phase for column chromatography separation to obtain 4-bromobutyl- Thioacetate 0.76g, yield rate is 31.0%;
[0065] 2) Weigh 0.26g (3.2mmol) of N-methylimidazole into a single-necked flask, add dichloromethane to dissolve, then add 0.61g (2....
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com