One-dimensional organic semiconductor nanowire/nanoribbon with fluorescence response to organic phosphorus-containing compound toxic gases and preparation method and application thereof
An organic semiconductor and toxic gas technology, applied in the field of one-dimensional organic semiconductor nanowires or nanobelts and their preparation, can solve the problems of low economic benefit, unreusable materials, long reaction time, etc., and achieve fast and sensitive response. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
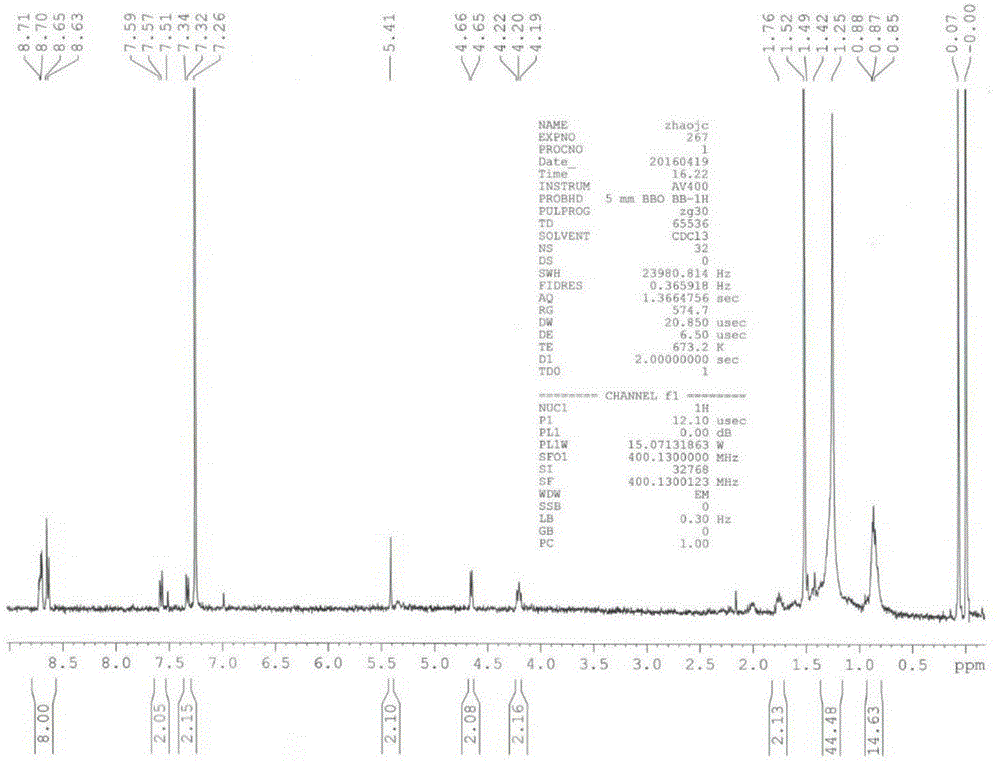
[0069] Prepare a peryleneimide derivative monomer containing perylene anhydride, which has the following molecular formula, with a 4-hydroxymethylbenzyl group at one end and a dodecyl group at the other end.
[0070]
[0071] (1) Mix 50 mg of perylene-3,4,9,10-tetracarboxylic dianhydride and 8 grams of imidazole and heat to 110°C to 130°C to dissolve, then slowly inject into the mixture solution relative to perylene-3, 4,9,10-Tetracarboxylic dianhydride molar excess dodecylamine solution was reacted for about 3 hours to obtain a reaction solution, and then 8 ml to 15 ml of ethanol and 8 ml to 15 ml of ethanol were added to the reaction solution Concentrated hydrochloric acid (36% in mass concentration) was stirred overnight; the product was taken out, washed with water until the pH was neutral, and dried;
[0072] (2) Take 50 mg to 100 mg of the product obtained after drying in step (1), add 8 g to 10 g of imidazole and 200 microliters of 4-hydroxymethylbenzylamine to it, and...
Embodiment 2
[0083] A peryleneimide derivative monomer containing perylene anhydride substituted with a 4-hydroxymethylbenzyl group at one end and a 5-aminononyl group at the other end having the following molecular formula was prepared.
[0084]
[0085] (1) Mix 50 mg of perylene-3,4,9,10-tetracarboxylic dianhydride and 8 grams of imidazole and heat to 130°C to dissolve, then slowly inject into the mixture solution relative to perylene-3,4,9 , 10-tetracarboxylic dianhydride molar excess 5-aminononanamine solution was reacted for about 3 hours to obtain a reaction solution, then in the reaction solution, add 10 milliliters of ethanol and 15 milliliters of concentrated hydrochloric acid (mass concentration is 36% ) and stirred overnight; the product was taken out, rinsed with water until the pH was neutral, and dried;
[0086] (2) Take 50 mg of the product obtained after drying in step (1), add 8 grams of imidazole and 200 microliters of 4-hydroxymethylbenzylamine to it, and react at a t...
Embodiment 3
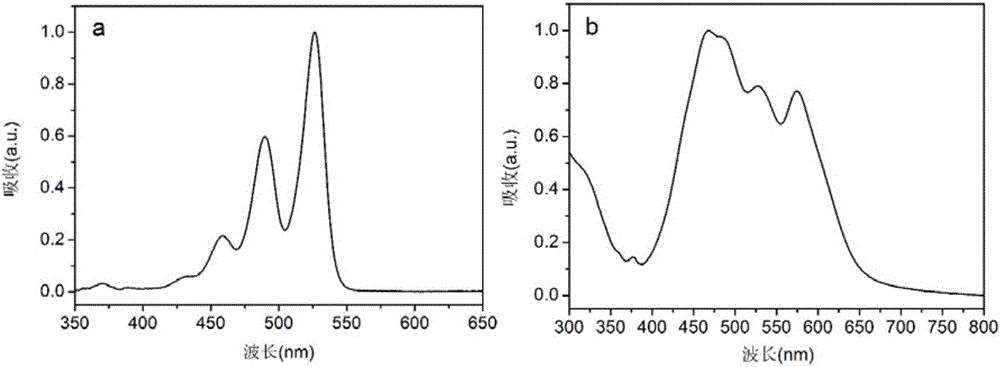
[0091] The film prepared in Example 1, which is self-assembled and braided by a plurality of red one-dimensional organic semiconductor nanowires to form a network structure, is used for fluorescence detection of toxic vapors of organophosphates and related organic phosphorus-containing compounds.
[0092] Expose the self-assembled and braided film material of a plurality of red one-dimensional organic semiconductor nanowires to form a network structure in diethylphosphoryl chloride (DCP) vapor, and use a 450 nm excitation light source to excite the self-assembly of a plurality of red one-dimensional organic semiconductor nanowires. Membranes that weave to form a network structure are assembled. A 10mL syringe was used to blow diethylphosphoryl chloride (DCP) gas of different concentrations to the surface of the porous membrane at a speed of 2mL / s, and the detection results showed obvious fluorescence enhancement. Compared Figure 7 , Figure 8 and Figure 9 , we can observe...
PUM
| Property | Measurement | Unit |
|---|---|---|
| length | aaaaa | aaaaa |
| width | aaaaa | aaaaa |
| length | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com