Hyaluronic acid crosslinked gel as well as preparation method and application thereof
A technology of hyaluronic acid and cross-linked gel, applied in medical science, prosthesis, tissue regeneration, etc., can solve the problems of unfavorable industrialization, difficult quantification of cross-linking degree, no obvious advantages, etc. The effect of gel properties
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0047] Follow the steps below to prepare sodium hyaluronate cross-linked gel:
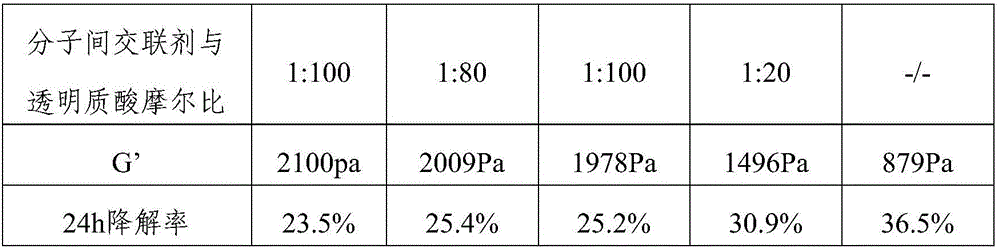
[0048] (1) Dissolve sodium hyaluronate with a molecular weight of 1 million Daltons in purified water to prepare a solution with a concentration of 1.5%, stir to fully dissolve sodium hyaluronate; slowly drop Add 2-chloro-1-methylpyridinium iodide (CMPI) so that the molar ratio of CMPI to sodium hyaluronate is 1:50, stir and mix thoroughly, and let stand at room temperature for 12 hours; use a dialysis bag to carry out the mixed solution Dialysis, regular replacement of purified water for dialysis, removal of CMPI, freeze-drying to obtain self-crosslinked products;
[0049] (2) Dissolving the self-crosslinking product in 0.5% NaOH to prepare a 4% solution. After heating to 40°C, the cross-linking agent 1,4-butanediol diglycidyl ether (BDDE) was slowly added dropwise, so that the molar ratio of added BDDE to initial sodium hyaluronate was 1:100. After fully stirring, let stand for 12 hours, remove...
Embodiment 2
[0051] Follow the steps below to prepare sodium hyaluronate cross-linked gel:
[0052] (1) Dissolve sodium hyaluronate with a molecular weight of 1 million Daltons in purified water to prepare a solution with a concentration of 2%, stir to fully dissolve sodium hyaluronate; slowly drop Add 2-chloro-1-methylpyridinium iodide (CMPI) so that the molar ratio of CMPI to sodium hyaluronate is 1:100. After fully stirring and mixing, let stand at room temperature for 12 hours; use a dialysis bag to carry out the mixed solution Dialysis, regular replacement of purified water for dialysis, removal of CMPI, freeze-drying to obtain self-crosslinked products;
[0053] (2) Dissolving the self-crosslinking product in 0.5% HCl to prepare a 4% solution. Slowly add carbodiimide (EDC) dropwise, so that the molar ratio of EDC and initial sodium hyaluronate is 1:80, stir well and let it stand for 12 hours; use ethanol for precipitation, and put the precipitated product into a dialysis bag for dia...
Embodiment 3
[0055] Follow the steps below to prepare sodium hyaluronate cross-linked gel:
[0056] (1) Dissolve sodium hyaluronate with a molecular weight of 1 million Daltons in 0.5% NaOH to prepare a solution with a concentration of 4%, stir to fully dissolve sodium hyaluronate; Slowly add 1,4-butanediol diglycidyl ether (BDDE) dropwise into the sodium hyaluronate solution, so that the molar ratio of BDDE to sodium hyaluronate is 1:100, stir well, stir at 50°C for 5h; ethanol Precipitate to remove unreacted cross-linking agent, dry to obtain cross-linked product;
[0057] (2) Dissolve the cross-linked product in purified water, configure it as a 1% solution, and slowly add 2-chloro-1-methylpyridinium iodide (CMPI) dropwise to the solution to make the CMPI and sodium hyaluronate The molar ratio is 1:50. After fully stirring and mixing, let stand at room temperature for 12 hours; use a dialysis bag to dialyze the mixed solution, replace the purified water for dialysis once regularly, rem...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com