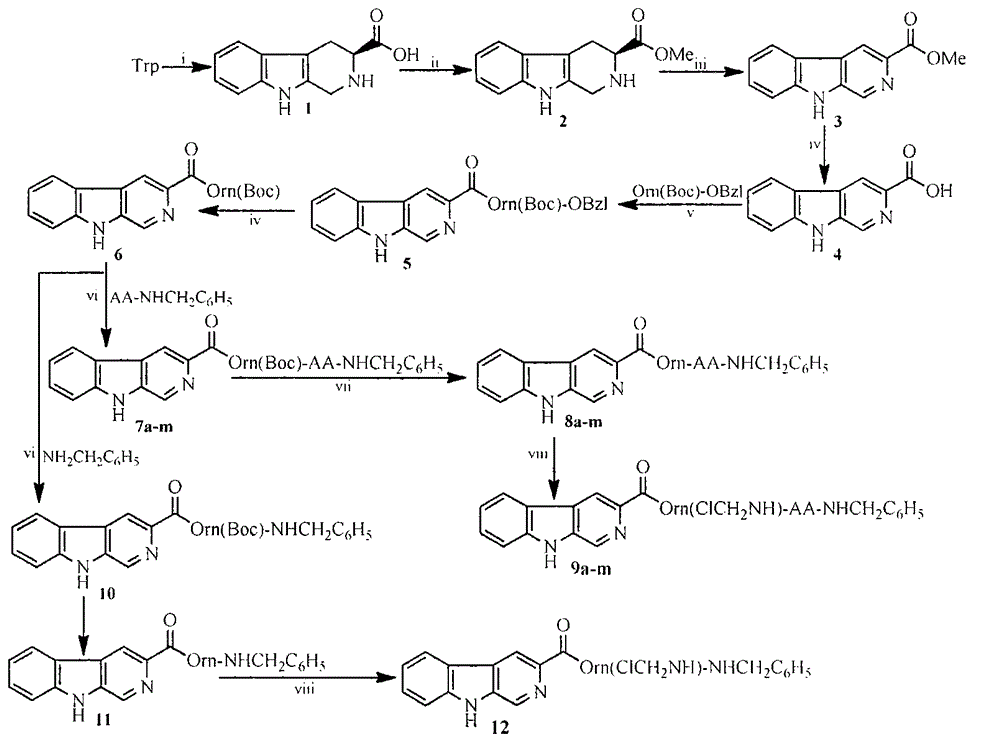
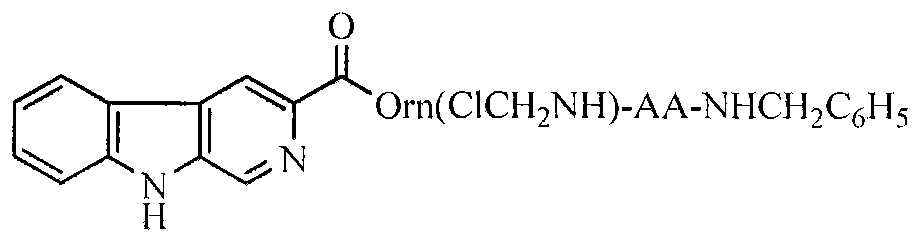
Carboline carboxylic-Orn(ClCH2NH)-AA-benzylamine, its synthesis, activity and application thereof
A technology of carboline and tetrahydrocarboline, applied in the application field of β-carboline-3-formyl-Orn-AA-NHCH2C6H5, in the preparation of antitumor drugs
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0022] Example 1 Preparation of (3s)-β-tetrahydrocarboline-3-carboxylic acid (1)
[0023]Slowly add 0.1 mL of concentrated sulfuric acid to 200 mL of water, stir for 10 minutes, add 2.5 g (12.2 mmol) of tryptophan to the obtained dilute sulfuric acid, and ultrasonically shake to completely dissolve the tryptophan. Then add 0.3 mL of 40% formaldehyde solution, react at room temperature for 6 hours, slowly add concentrated ammonia water to the reaction solution under ice bath, and adjust the pH to 6. The reaction solution became turbid and was filtered to obtain 2.2 g (85%) of the title compound as a colorless powder, ESI-MS (m / z): 205 [M+H] + .
Embodiment 2
[0024] Example 2 Preparation of (3s)-β-tetrahydrocarboline-3-carboxylic acid methyl ester (2)
[0025] Stir the solution of 18mL methanol and 1.2mL thionyl chloride in an ice bath for 30min, then add 750mg (3.2mmol) (3s)-β-tetrahydrocarboline-3-carboxylic acid, gradually return to room temperature and continue Reaction 24h. TLC (dichloromethane:methanol, 20:1) detected the disappearance of the reaction starting material. The reaction mixture was concentrated to dryness under reduced pressure, methanol was added to the residue, and concentrated to dryness under reduced pressure. This operation was repeated 3 times. The residue was added with diethyl ether and concentrated under reduced pressure to obtain 250 mg (35%) of the title compound as a yellow powder. ESI-MS(m / e): 231[M+H] + .
Embodiment 3
[0026] Embodiment 3 prepares β-carboline-3-carboxylic acid methyl ester (3)
[0027] Dissolve 230 mg (1 mmol) methyl tetrahydrocarboline-3-carboxylate in 50 mL of acetone, slowly add 100 mg (1 mmol) potassium permanganate to the solution under ice-cooling, and react at room temperature for 4 h. TLC (dichloromethane:methanol, 20:1) detected the end of the reaction. The reaction solution was filtered, the filtrate was concentrated under reduced pressure and spin-dried, the filter cake was dissolved with acetone, filtered under reduced pressure, the filtrate was concentrated to dryness, this operation was repeated 3 times, and the filtrate was combined to obtain a yellow oil separated and purified by column chromatography (dichloromethane: Methanol, 40:1), afforded 60 mg (20%) of the title product as a yellowish solid, ESI-MS (m / e): 227 [M+H] + .
PUM
| Property | Measurement | Unit |
|---|---|---|
| Cell number | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com