Aspartate-β-semialdehyde dehydrogenase mutant and its application
A semialdehyde dehydrogenase and aspartic acid technology, applied in the field of aspartate-β-semialdehyde dehydrogenase mutants, can solve the problems of affecting the rapid growth of bacterial cells, energy consumption and cell metabolic pressure, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0125] Example 1, construction of a single-point saturation mutation library
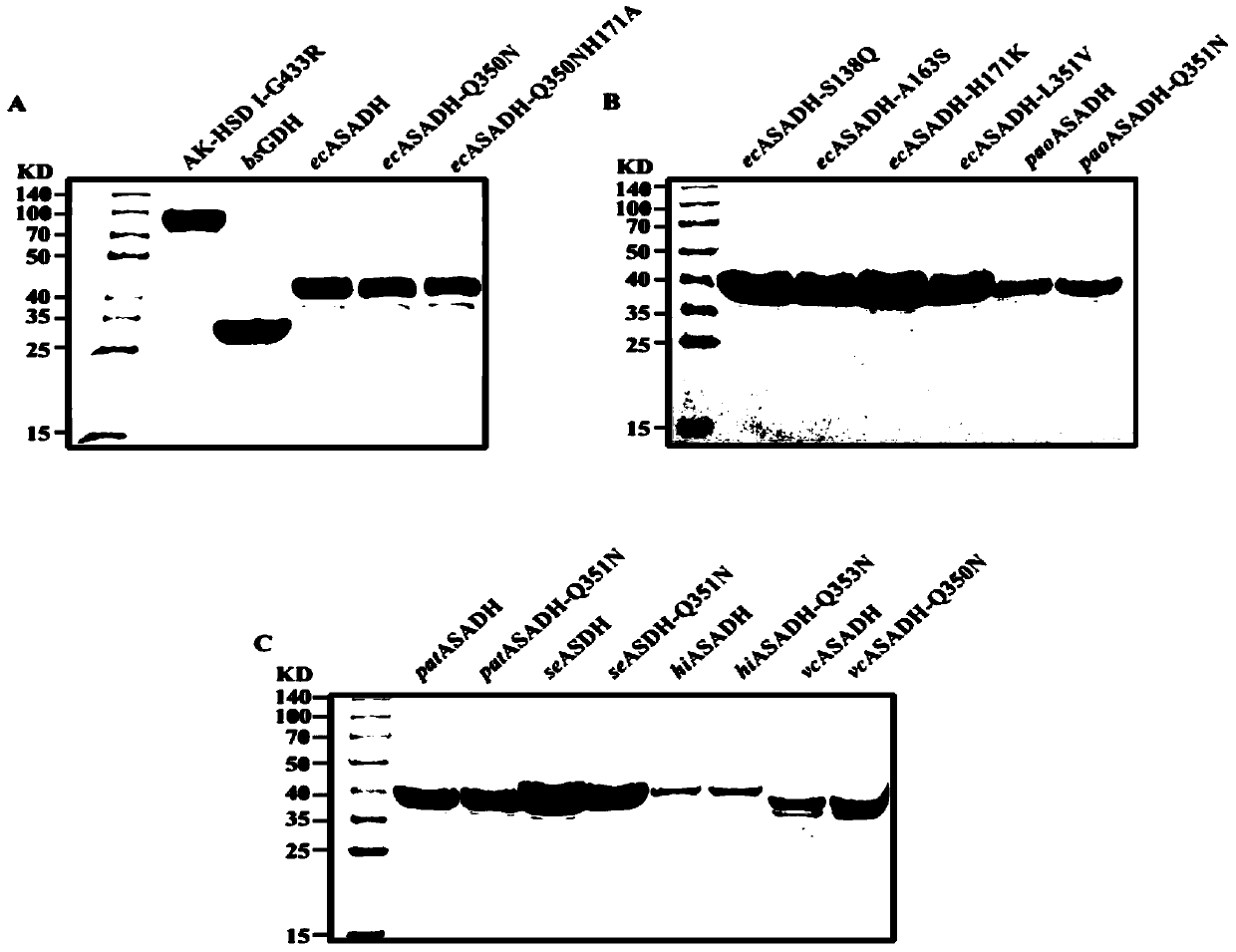
[0126] Using ASADH derived from E.coli, the asd gene was amplified from the genome of MG1655, and 6 His were added to its N-terminus, and then linked into pET28a vector for expression. The wild-type ASADH derived from Escherichia coli was obtained by purifying with a nickel column.
[0127] The three-dimensional structure of ASADH protein derived from Escherichia coli was searched through the RCSB PDB database, and two different forms of ASADH were retrieved from the data, including the open form and the closed form. The structure whose PDB ID is 1GL3 is a three-dimensional complex of ASADH, NADP and its substrate analogs, and this structure is used as the basis for subsequent analysis.
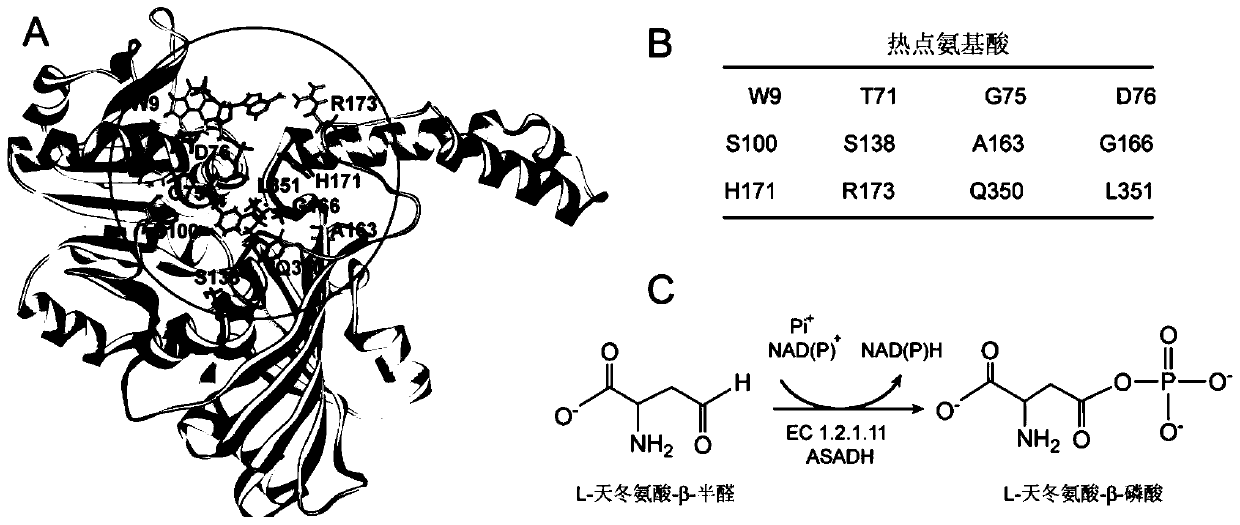
[0128] Use Molegro Molecular Viewer (Thomsen, R. et al., Journal of medicinalchemistry 49, 3315-3321) to analyze the crystal structure of ASADH (ecASADH) derived from Escherichia coli, select NADP + binding activ...
Embodiment 2
[0131] Example 2. Identification of cofactor-dependent enzymatic properties of ecASADH-Q350N and ecASADH-Q350N / H171A
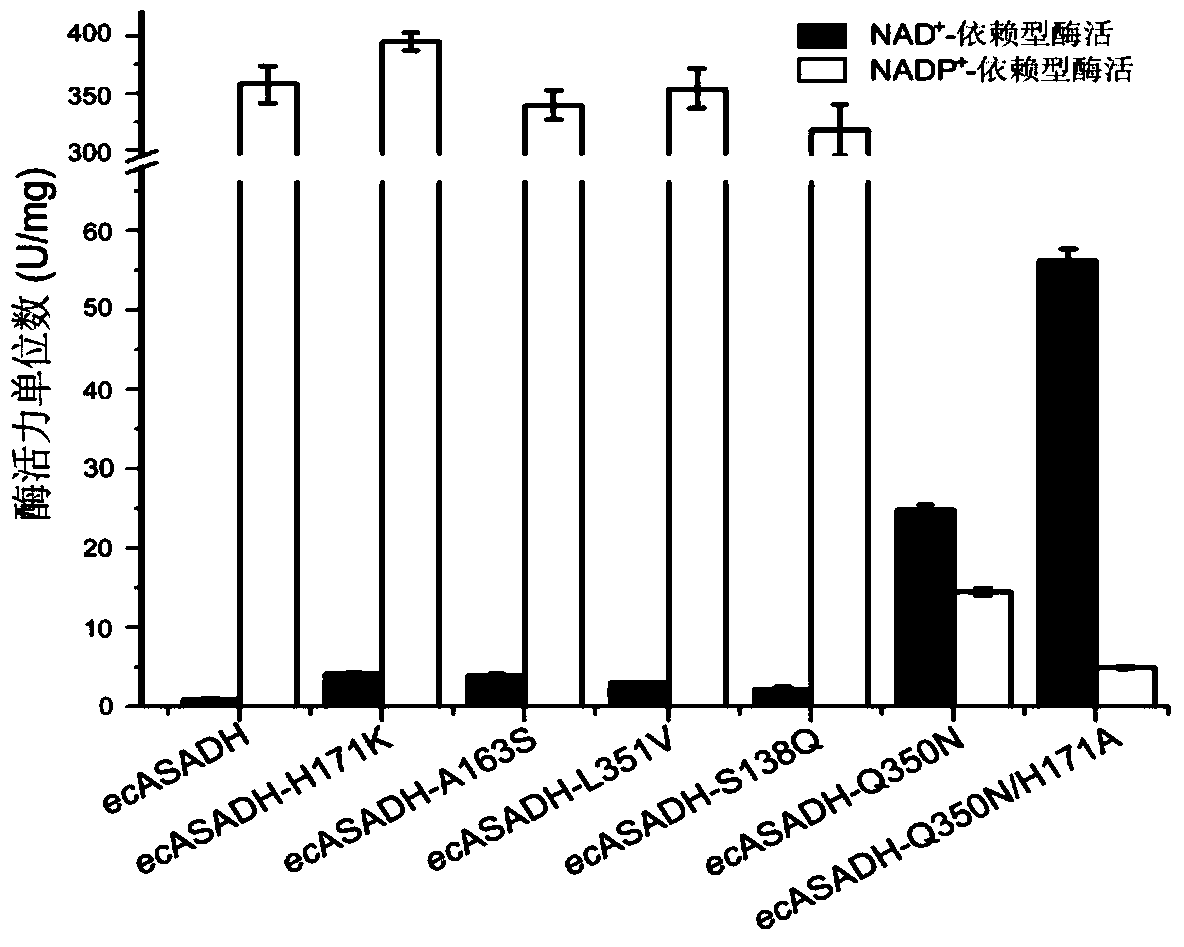
[0132] NAD + The two mutant proteins with the highest -dependent enzyme activity were tested for their enzymatic properties, and the results are shown in Table 2. The results of the assay showed that the two mutant proteins had an effect on NAD + The affinity of K m values are reduced by a factor of 4.6 and 35, respectively. At the same time, the catalytic efficiency was improved, compared with the wild-type protein, the effect on NAD + conversion number k cat The values are increased by 9 and 12 times, respectively.
[0133] NADP + The affinity of the binding mutant protein to NADP was also greatly improved + The decline of catalytic performance, the inventor speculates that after protein mutation, it strengthens the ability to NADP + binding force, leading to NADP + The dissociation becomes rate-limiting, resulting in decreased responsiveness. ...
Embodiment 3
[0136] Embodiment 3, wild-type ecASADH and mutant protein pair cofactor NADP + Determination of binding capacity
[0137] NADP was determined by isothermal titration calorimetry (ITC) + Dissociation constant k with ecASADH, ecASADH-Q350N, ecASADH-Q350N / H171A d . The result is as Figure 4 , it can be seen from the change of the dissociation constant, which is consistent with the enzymatic parameters, and the protein is mutated with NADP + The binding force has been greatly improved. Excessively high binding force may lead to the slow dissociation of the product NADPH and affect the ability of NADP + catalytic ability.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com