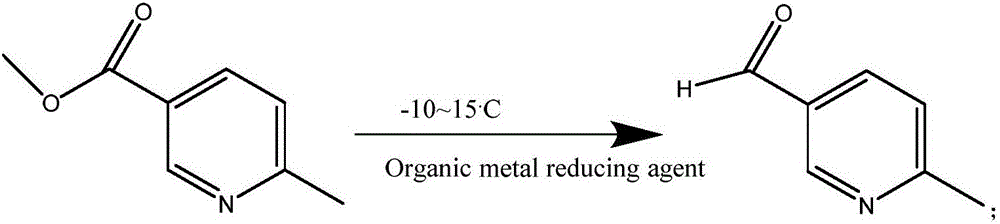Synthesis method of 2-methyl-5-vinylpyridine
A technology of vinylpyridine and synthesis method, applied in the direction of organic chemistry, etc., can solve the problems of large amount of chemical raw materials, complex synthesis process, unfriendly environment, etc., achieve wide application value, simplify the treatment process, and reduce the effect of experimental steps
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
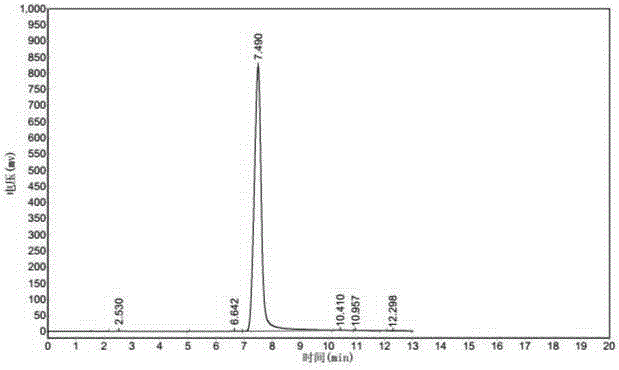
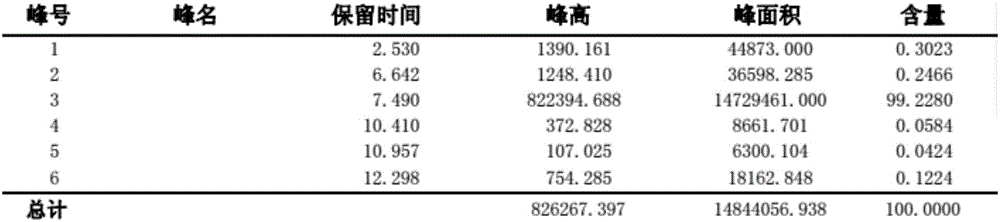
Image
Examples
Embodiment 1
[0036] 1) Take 350ml of metal-organic reducing reagent and place it in a 1000ml four-necked bottle, cool down to -10°C, dissolve 65 grams of 6-methylnicotinic acid methyl ester in 200ml of ether organic solvent and slowly add it dropwise into the four-necked bottle , the dropping time should not be less than 2h, and the whole system should replace the air with nitrogen before dropping;
[0037] 2) Put 173g of methyl bromide triphenylphosphonium salt into a 2L four-necked bottle, add 400ml of ether organic solvent, when the temperature of the solvent in the bottle drops below 5°C, add 57g of potassium tert-butoxide in batches, and wait until the reaction is over Continue to stir for 1h and set aside;
[0038]3) Slowly add the reaction solution obtained in 1) to 2), and control the rate of addition. During the reaction, the temperature of the reaction solution will rise, and the temperature should not exceed 10°C. After the addition is completed, continue the reaction for 30 min...
Embodiment 2
[0040] The inventive method comprises:
[0041] 1) At a temperature of -10 to 15°C, dissolve methyl 6-methylnicotinate in an ether organic solvent to obtain solution A, slowly add solution A to the metal organic reducing reagent, and add solution A dropwise The time to the metal organic reducing reagent is not less than 2h, and the reaction environment during the dropwise addition adopts a nitrogen atmosphere to obtain solution B; the ether organic solvent is diethyl ether, and the metal organic reducing reagent is organometallic lithium, 6-methyl nicotinic acid The ratio of the quality of the methyl ester to the volume of the ether organic solvent is 1:3, the ratio of the quality of 6-methyl nicotinic acid methyl ester to the volume of the metal organic reducing agent is 1:1;
[0042] 2) Take methyl bromide triphenylphosphonium salt and add ether organic solvent to obtain solution C, add potassium tert-butoxide to solution C at a temperature below 5°C for reaction, stir after...
Embodiment 3
[0045] The inventive method comprises:
[0046] 1) At a temperature of -10 to 15°C, dissolve methyl 6-methylnicotinate in an ether organic solvent to obtain solution A, slowly add solution A to the metal organic reducing reagent, and add solution A dropwise The time to the metal organic reducing reagent is not less than 2h, and the reaction environment during the dropwise addition adopts a nitrogen atmosphere to obtain solution B; the ether organic solvent is methyl tert-butyl ether, and the metal organic reducing reagent is organometallic lithium and organic Metal copper, the ratio of the quality of 6-methyl nicotinic acid methyl ester and the volume of ether organic solvent is 1:4, the ratio of the quality of 6-methyl nicotinic acid methyl ester and the volume of metal organic reducing reagent is 1 :1.2;
[0047] 2) Take methyl bromide triphenylphosphonium salt and add ether organic solvent to obtain solution C, add potassium tert-butoxide to solution C at a temperature bel...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com