Amino-protected 3-hydroxy adamantane glycine benzothiazole-2-thiol active ester as well as preparation method and application thereof
An adamantane glycine and amino protection technology, which is applied in the field of intermediates for preparing saxagliptin, can solve the problems of inapplicability of amino protecting groups, inability to recycle, harsh reaction conditions, etc., and achieves improved drug purity, mild reaction, adaptable effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0054] Compound Ⅰ (PG=Boc), chemical structural formula:
[0055] .
[0056] Example 1 Compound I Melting point: 139.8~142.3 °C;
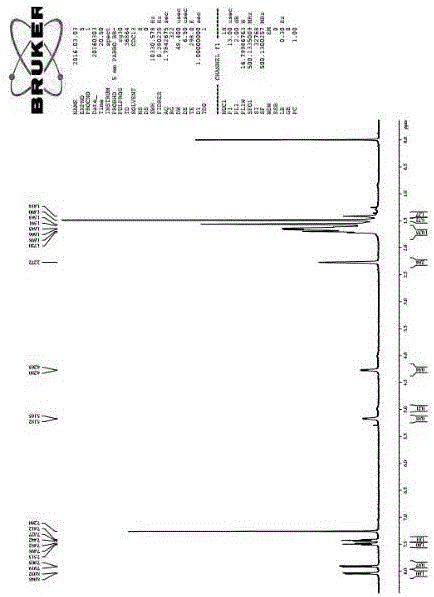
[0057] The characteristics of the NMR spectrum are: 1 H NMR (500 MHz, CDCl 3 ) δ: 1.41~1.72 (m, 21H),2.27 (s, 2H),4.26~4.28 (d, J=8.5 Hz, 1H), 5.16~5.18 (d, J=8.5 Hz, 1H), 7.41~7.44 (m, 1H),7.48~7.51 (m, 1H), 7.90 (d, J=8 Hz, 1H), 8.03 (d, J=8Hz, 1H), such as figure 1 shown.
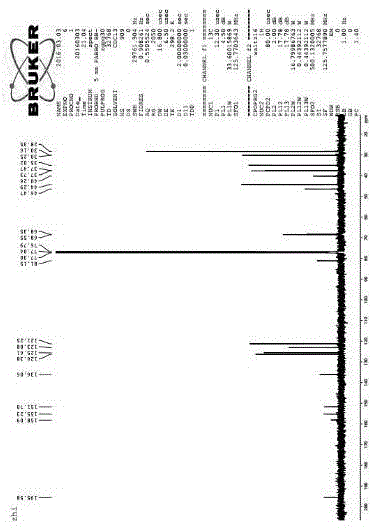
[0058] 13 C NMR (125 MHz, CDCl 3 ) Δ: 28.35, 30.16, 30.25, 35.02, 37.47, 37.73,40.26, 44.29, 46.47, 68.35, 68.55, 81.15, 121.25, 125.61, 126.38,136.06, 155.23, 158.09, 195.58, figure 2 shown.
Embodiment 2
[0059] The preparation of embodiment 2 saxagliptin intermediate
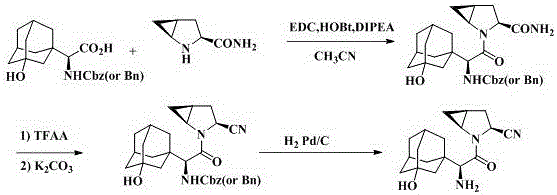
[0060] Step 1: Synthesis of amino-protected 3-hydroxyadamantaneglycine benzothiazole-2-thiol active ester intermediate (Ⅰ) through amino-protected 3-hydroxyadamantaneglycine (Ⅱ) and dibenzothiazole disulfide (Ⅲ) ) method: add compound II and compound III to a three-necked flask, stir with an aprotic solvent, control the temperature at 10 °C, add an organic base, cool down to 0 °C, add an organic phosphine reagent dropwise, remove the ice bath and continue stirring, The color of the solution changed from white turbidity to light yellow and clear. Stirring was continued at room temperature, and solids precipitated out of the solution. Stand overnight at room temperature, and filter with suction to obtain a white solid. The product was washed with sodium carbonate aqueous solution and suction filtered to obtain the amino-protected 3-hydroxyadamantylglycine benzothiazole-2-thiol active ester compound.
[0061] Ste...
Embodiment 3
[0065] Amino-protected 3-hydroxyadamantane glycine (compound Ⅱ, PG=Boc) (10 g, 30.7 mmol), dibenzothiazole disulfide (compound Ⅲ) (11.3 g, 33.7 mmol), acetonitrile (50 mL), Add it to a 100 mL reaction bottle in turn, stir at room temperature, the solution is white and turbid. Control the temperature at 10°C, add triethylamine (5.2 mL) dropwise, when the temperature continues to drop to 0°C, add triethyl phosphite (6.2 g, 36.9 mmol) dropwise, the solution is still white and turbid after the dropwise addition, remove the ice and continue stirring , the color of the solution changed from white turbidity to light yellow and clear. Stirring at room temperature gradually precipitated solids in the solution. Stand overnight at room temperature, and filter with suction to obtain a white solid. The obtained white solid was washed with 10% aqueous sodium carbonate solution, filtered with suction, and dried to obtain 12.5 g of compound (Compound I, PG=Boc), with a yield of 85.6%.
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com