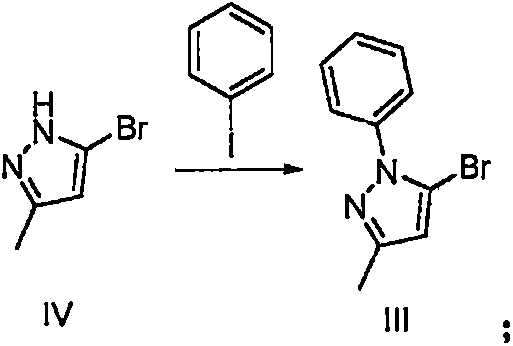
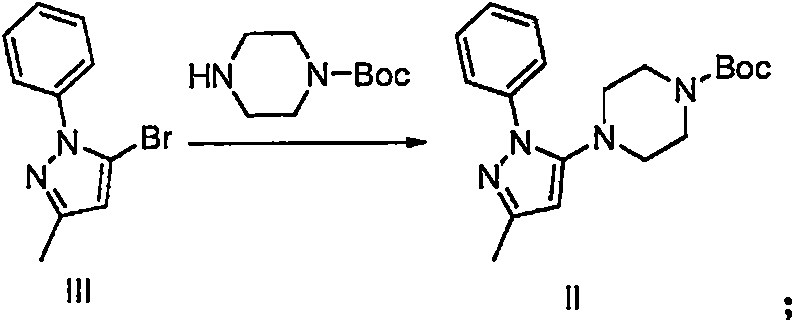
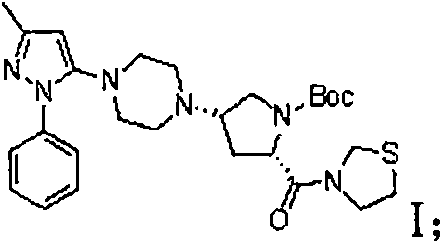
Method for preparing hydrobromic acid teneligliptin
A technology of hydrobromic acid and glutamic acid, applied in the production of bulk chemicals, organic chemistry, etc., can solve the problems of high cost, low yield and purity, and achieve the effect of low cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0044] This embodiment relates to a preparation method of Teliliptin hydrobromide, which includes the following steps:
[0045] S1: Add glutamic acid to the reaction tank, heat the oil bath to 220-290°C, stir and dehydrate, react at 130-180°C for 40-70 minutes, put it in water, cool, and filter to obtain crude pyroglutamic acid. Add water to the crude pyroglutamic acid to saturate it at 40-70°C, cool to 30-40°C to crystallize, separate the crystals, wash with detergent, and dry to obtain a refined pyroglutamic acid;
[0046] S2: Add absolute ethanol to the flask, cool to below -5°C with an ice-salt bath, add the catalyst and the pyroglutamic acid obtained in step S1, stir and slowly raise the temperature to react at 20-45°C for 24 to 48 hours; in use Adjust the pH to 7-8 with alkali, collect the filtrate by filtration, wash the filter residue with detergent, combine the washing and filtrate and concentrate to obtain a crude product, which is purified by distillation under the condi...
Embodiment 2
[0062] This embodiment relates to a preparation method of Teliliptin hydrobromide, which includes the following steps:
[0063] S1: Add glutamic acid to the reaction tank, heat the oil bath to 220-290°C, stir and dehydrate, react at 130-180°C for 40-70 minutes, put it in water, cool, and filter to obtain crude pyroglutamic acid. Add water to the crude pyroglutamic acid to saturate it at 40-70°C, cool to 30-40°C to crystallize, separate the crystals, wash with detergent, and dry to obtain a refined pyroglutamic acid;
[0064] S2: Add absolute ethanol to the flask, cool to below -5°C with an ice-salt bath, add the catalyst and the pyroglutamic acid obtained in step S1, stir and slowly raise the temperature to react at 20-45°C for 24 to 48 hours; in use Adjust the pH to 7-8 with alkali, collect the filtrate by filtration, wash the filter residue with detergent, combine the washing and filtrate and concentrate to obtain a crude product, which is purified by distillation under the condi...
Embodiment 3
[0080] This embodiment relates to a preparation method of Teliliptin hydrobromide, which includes the following steps:
[0081] S1: Add glutamic acid to the reaction tank, heat the oil bath to 220-290°C, stir and dehydrate, react at 130-180°C for 40-70 minutes, put it in water, cool, and filter to obtain crude pyroglutamic acid. Add water to the crude pyroglutamic acid to saturate it at 40-70°C, cool to 30-40°C to crystallize, separate the crystals, wash with detergent, and dry to obtain a refined pyroglutamic acid;
[0082] S2: Add absolute ethanol to the flask, cool to below -5°C with an ice-salt bath, add the catalyst and the pyroglutamic acid obtained in step S1, stir and slowly raise the temperature to react at 20-45°C for 24 to 48 hours; in use Adjust the pH to 7-8 with alkali, collect the filtrate by filtration, wash the filter residue with detergent, combine the washing and filtrate and concentrate to obtain a crude product, which is purified by distillation under the condi...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com