Cell culture method for improving DC-CIK (dendritic cell-cytokine-induced killer) cell killing ability
A technology of DC-CIK and cell culture, which is applied in the direction of cell culture active agent, co-culture, tissue culture, etc., can solve the problems of restricting technology development, DC-CIK cell lethality not being achieved, reducing DC-CIK cell lethality, etc. , to achieve the effect of stimulating lethality and improving the effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0021] Example 1: Culture of DC-CIK cells
[0022] Methods for isolation and culture of DC cells and CIK cells:
[0023] Collect 20mL of peripheral blood from healthy volunteers, dilute it with pre-cooled PBS 1:1, slowly add the upper layer of lymphocyte separation medium, centrifuge at 650g, 4°C for 20min, collect the white cell layer, separate mononuclear cells, and resuspend the cells in RPMI 1640 medium Place at 37°C, 5% CO 2 Incubate for 2 hours in the incubator. Culture of DC cells: Collect adherent cells after 2 hours of culture, add 1000U / mL GM-CSF and 1000U / mL IL-4 to RPMI1640 medium containing 10% fetal bovine serum, and store at 37°C, 5% CO 2 Cultured in an incubator, supplemented with GM-CSF and IL-4 after changing the medium, continued to culture for 6 days, and added 100ng / mL TNF-α to the medium on 5 days. Cultivation of CIK cells: collect the suspension cells in monocytes after 2 hours of culture, add 1000U / mL INF-γ to RPMI 1640 medium containing 10% fetal bo...
Embodiment 2
[0026] Embodiment 2: the contrast of embodiment 1, do not add eliciting peptide during mixed culture
[0027] Methods for isolation and culture of DC cells and CIK cells:
[0028] Collect 20mL of peripheral blood from healthy volunteers, dilute it with pre-cooled PBS 1:1, slowly add the upper layer of lymphocyte separation medium, centrifuge at 650g, 4°C for 20min, collect the white cell layer, separate mononuclear cells, and resuspend the cells in RPMI 1640 medium Place at 37°C, 5% CO 2 Incubate for 2 hours in the incubator. Culture of DC cells: Collect adherent cells after 2 hours of culture, add 1000U / mL GM-CSF and 1000U / mL IL-4 to RPMI1640 medium containing 10% fetal bovine serum, and store at 37°C, 5% CO 2 Cultured in an incubator, supplemented with GM-CSF and IL-4 after changing the medium, continued to culture for 6 days, and added 100ng / mL TNF-α to the medium on 5 days. Cultivation of CIK cells: collect the suspension cells in monocytes after 2 hours of culture, add...
Embodiment 3
[0031] Example 3: Cytotoxicity
[0032] The cytotoxicity of the DC-CIK cells prepared in Examples 1 and 2 were tested respectively. Using K562 cells as target cells, use complete medium (RPMI 1640 containing 10% fetal bovine serum) to adjust the cell concentration to 10 5 cells / mL, using the DC-CIK cells prepared in Examples 1 and 2 as effector cells, adding effector cells and target cells into a 96-well plate according to the effect target ratio of 10:1, at 37°C, 5% CO 2 Cultured in the incubator for 48h. Cell viability was detected by MTT detection kit.
[0033] Killing activity (%)=[1-(mean value of test wells-mean value of effect control wells) / mean value of control wells of target cells]×100%.
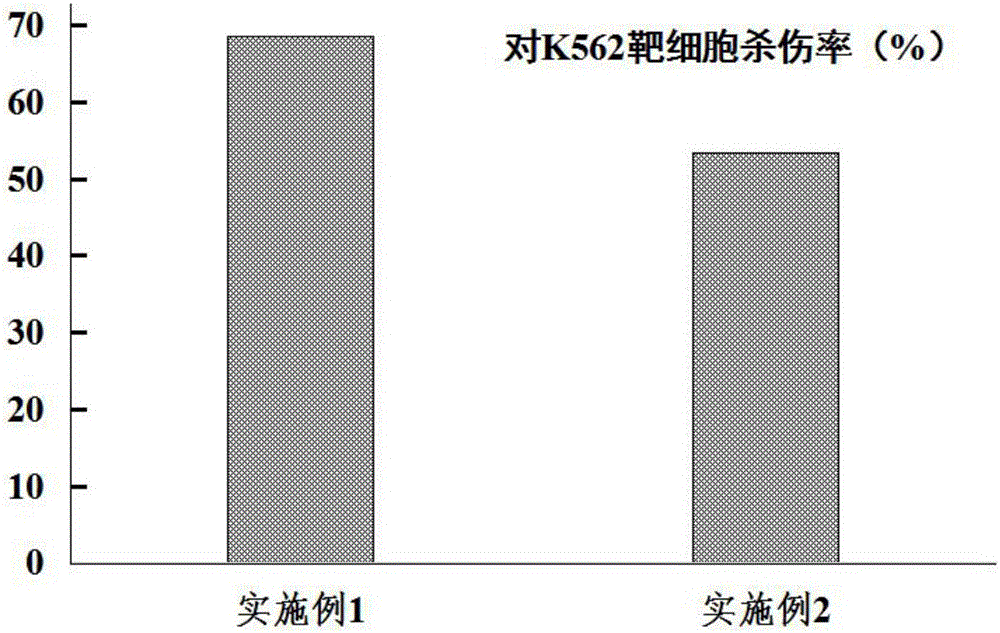
[0034] The results are shown in Table 1 and figure 1 .
[0035] Table 1 DC-CIK cell killing rate (%) to K562 target cell
[0036] Example 1 Example 2 Kill rate (%) 68.55±9.45 53.42±8.34
[0037] The results show that the method of the present inventio...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com