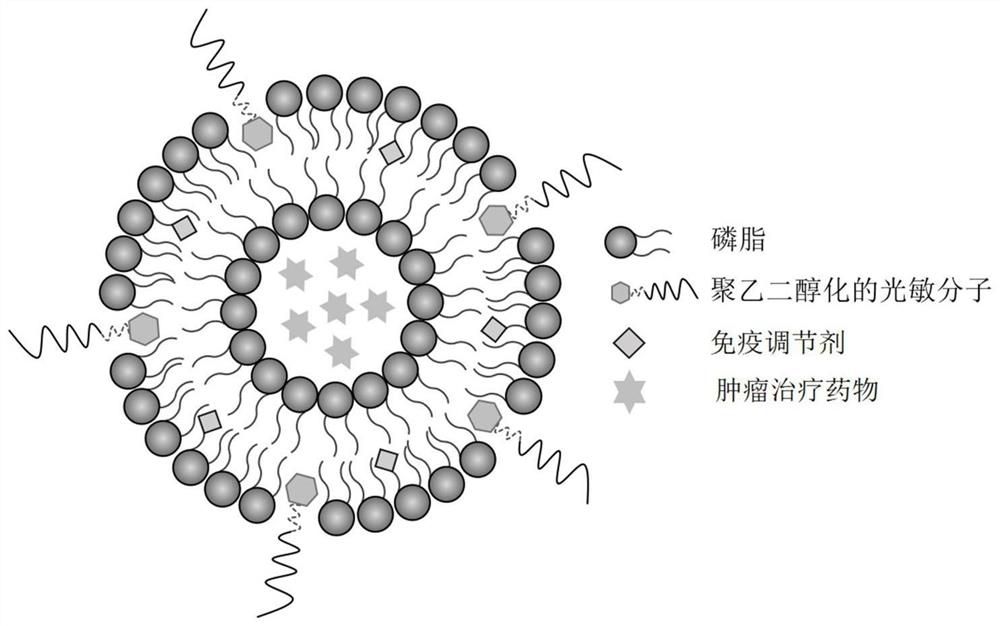
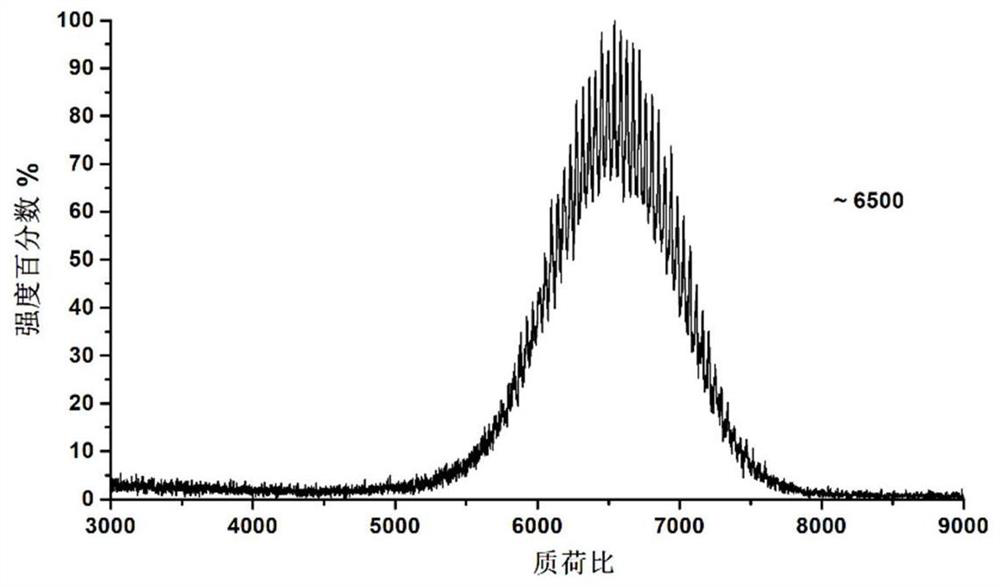
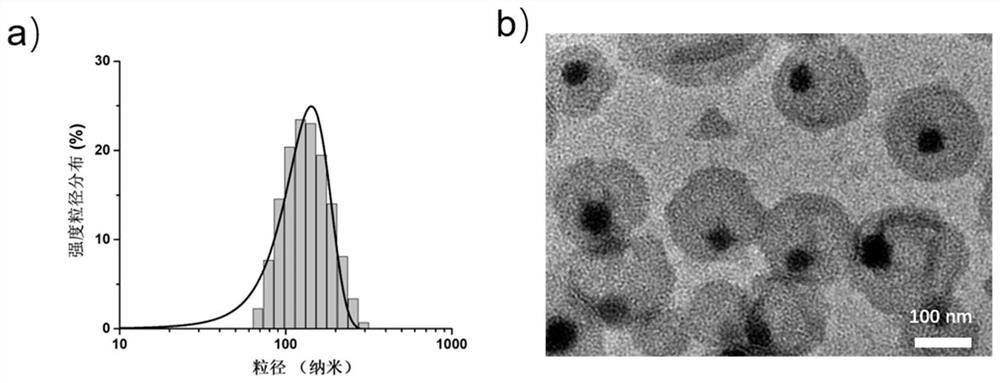
Composite liposome, preparation method and application thereof
A compound lipid and plastid technology, applied in the field of medicine and chemical industry, can solve problems such as difficult to effectively control tumor growth and metastasis process, achieve the effects of promoting drug action, inhibiting growth and proliferation, and improving tumor immune microenvironment
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation Embodiment 1
[0068] Preparation Example 1: Synthesis of Carboxylated Palmitoyl Lysolecithin 1
[0069]
[0070] P-LysoPC (300mg, 0.6mmol) and succinic anhydride (120mg, 1.2mmol) were dissolved in 5.0mL of dichloromethane, and N,N-dimethylaminopyridine (150mg, 1.2mmol) was added thereto, heated to After reacting at 40° C. for 36 h, the solvent was spun off, and carboxylated palmitoyl lysolecithin 1 (325.7 mg) was separated by a reverse-phase column with a yield of 90.5%.
preparation Embodiment 2
[0071] Preparation Example 2: Synthesis of JQ1-phospholipid conjugate JPC
[0072]
[0073] (S)-(+)-2-(4-(4-chlorophenyl)-2,3,9-trimethyl-6H-thieno[3,2-F][1,2,4] Triazolo[4,3-A][1,4]diazepine-6-yl) tert-butyl acetate (JQ1) (200mg, 0.44mmol) was dissolved in 10.0mL 40% (v / v%) tri In dichloromethane solution of fluoroacetic acid, react at room temperature for 3h. After the reaction was completed, the solvent was spun off, and compound 2 (168 mg) was obtained by column separation with a yield of 95.8%.
[0074] Compound 2 (160 mg, 0.4 mmol) was dissolved in 5.0 mL of dichloromethane, and N,N-dimethylaminopyridine (146.8 mg, 1.2 mmol), 1-(3-dimethylaminopropyl)- 3-Ethylcarbodiimide hydrochloride (230mg, 1.2mmol) and N,N-diisopropylethylamine (198.4μL, 1.2mmol), after activation in ice bath for 2h, add 2-hydroxyethyl di Sulfide (144μL, 1.2mmol), reacted at room temperature for 24h. After the reaction was completed, the solvent was spun off, and compound 3 (165 mg) was obtain...
preparation Embodiment 3
[0076] Preparation Example 3: IR-1061-GPLGLAG-PEG 5k Synthesis
[0077]
[0078] Fmoc-GPLGLAG (250mg, 0.3mmol), 1-(3-dimethylaminopropyl)-3-ethylcarbodiimide hydrochloride (72.4mg, 0.37mmol), 1-hydroxybenzotriazole ( 51.0mg, 0.37mmol) was dissolved in 2.0mL of anhydrous DMF, after activation at room temperature for 1h, mPEG was added 5k -NH 2 (500mg, 0.1mmol), react at room temperature for 24h. After the reaction was completed, the solvent was removed by ethanol dialysis, and the ethanol was spin-dried to obtain compound 4 (430 mg), with a yield of 74.1%.
[0079] Compound 4 (425 mg, 0.073 mmol) was dissolved in 5.0 mL of 20% (v / v%) 4-methylpiperidine in anhydrous DMF, and reacted at room temperature for 24 h. After the reaction was completed, the solvent was removed by ethanol dialysis, and the ethanol was spin-dried to obtain compound 5 (370 mg), with a yield of 90.2%.
[0080] Carboxylated IR-1061 (50mg, 0.062mmol), 1-(3-dimethylaminopropyl)-3-ethylcarbodiimide hydr...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com