pH redox dual sensitive PAMAM (Polyamidoamine) targeted nano drug delivery carrier and preparation method thereof
A nano drug delivery carrier and sensitive technology, which is applied in the direction of pharmaceutical formulations, medical preparations with non-active ingredients, medical preparations containing active ingredients, etc., can solve the problems of reducing drug release and reducing the anti-tumor effect of drugs, and achieve reduction Effects of uptake, reduction of cytotoxicity and hemolytic toxicity, and prolongation of residence time
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
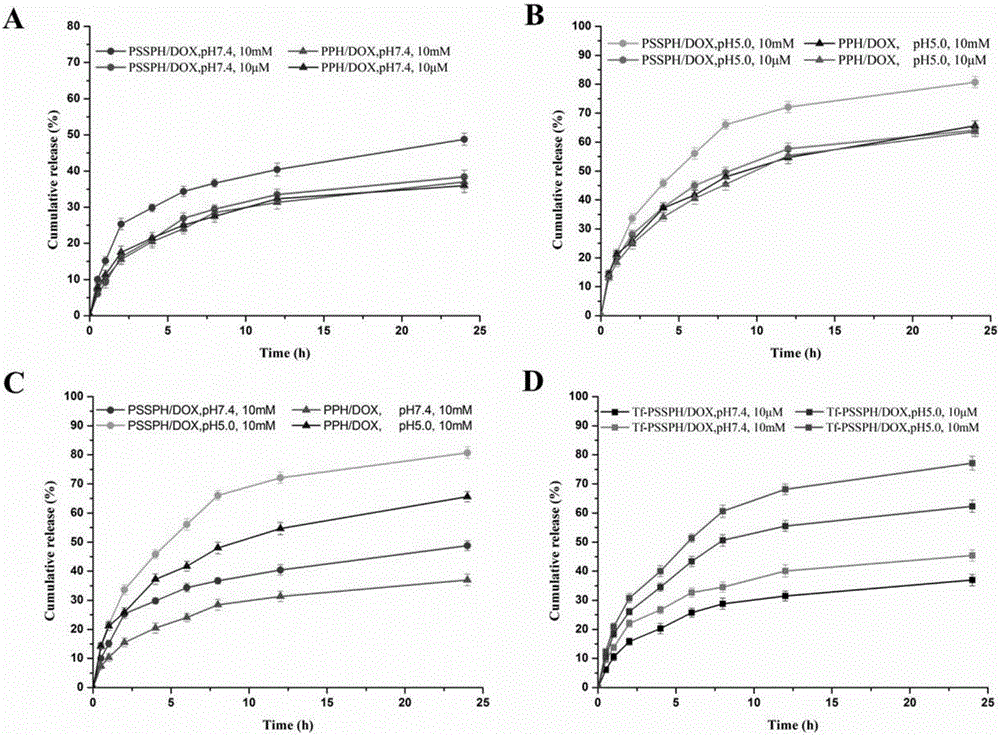
Examples
Embodiment 1
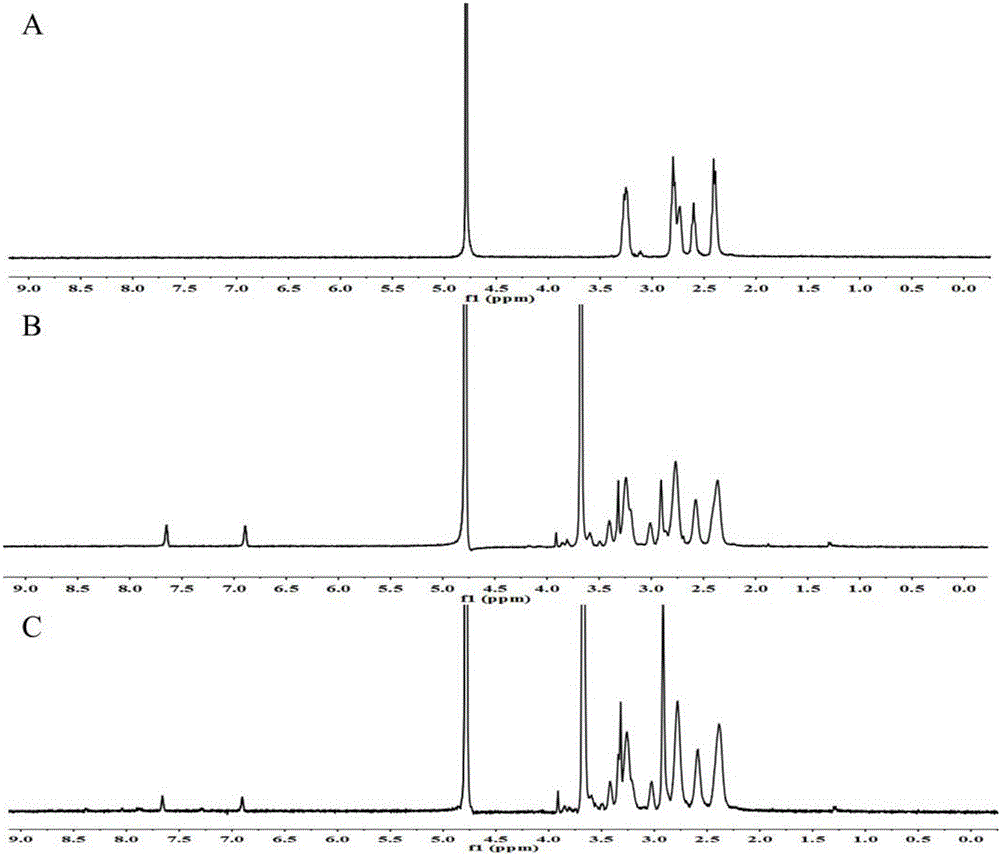
[0033] Accurately weigh Fmoc-His(trt)-OH, 1-hydroxy-benzo-triazole (HOBt), benzotriazole-N,N,N,N-tetramethyluronium hexafluorophosphate (HBTU ), N,N-diisopropylethylamine (DIPEA) and PAMAM were dissolved in anhydrous dimethylformamide (DMF) according to the reaction molar ratio of 64:64:64:64:1, under nitrogen protection, at room temperature and protected from light React for 12 hours; precipitate the reaction product with cold ether, wash, centrifuge, then redissolve in piperidine / DMF (v / v, 30 / 70) solution, and continue the reaction for 6 hours; precipitate the product again with cold ether, then dissolve in trifluoroacetic acid / Triisopropylsilane / H 2 In O solution, reacted for 12 hours; finally precipitated with cold ether, redissolved in DMF, dialyzed in deionized water for 24 hours, and freeze-dried to obtain the final product PAMAM-His.
[0034] The synthesized product PAMAM-His and SPDP were neutralized by adding methanol solution at a molar ratio of 1:32, adding triet...
Embodiment 1、2、3
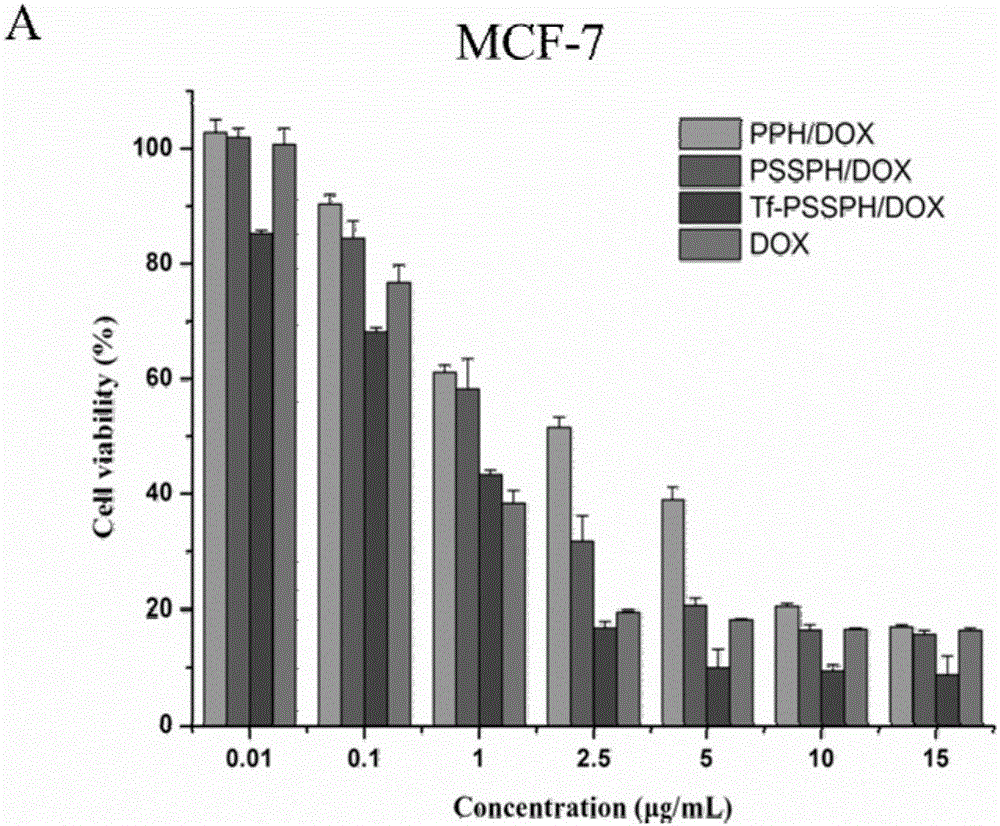
[0055] Example 1, 2, 3, free DOX to the IC of MCF-7 and HEPG2 cells 50 The value is as follows
[0056]
[0057] From the above table, image 3 and Figure 4 It can be seen that each complex has a certain anti-tumor effect on MCF-7 and HEPG2 cells. The cytotoxicity of the different complexes to both cells showed a dependence increase with the increase of DOX concentration. At the same dosage, the Tf-PSSPH / DOX drug-loaded complex exhibited the strongest cytotoxicity, indicating that Tf linking can significantly enhance the cytotoxicity of the complex. For MCF-7 cells, the IC50 of the Tf-PSSPH / DOX drug-loaded complex was 0.297 μg / mL, and its cytotoxicity was that of the PPH / DOX drug-loaded complex (1.873 μg / mL) and the PSSPH / DOX drug-loaded complex (1.114μg / mL) 6.31 and 3.75 times; for HepG2 cells, the IC50 of Tf-PSSPH / DOX drug-loaded complex is 0.243μg / mL, and its cytotoxicity is respectively that of PSSPH / DOX drug-loaded complex (0.743μg / mL) and 3.06 and 1.85 times of...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com