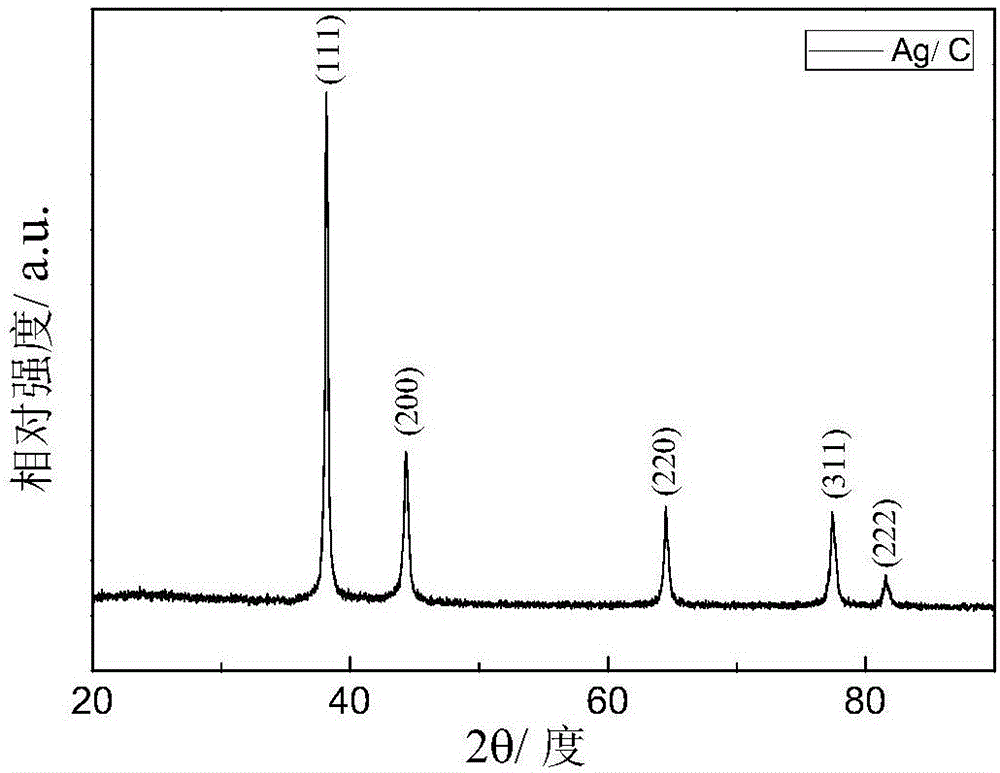
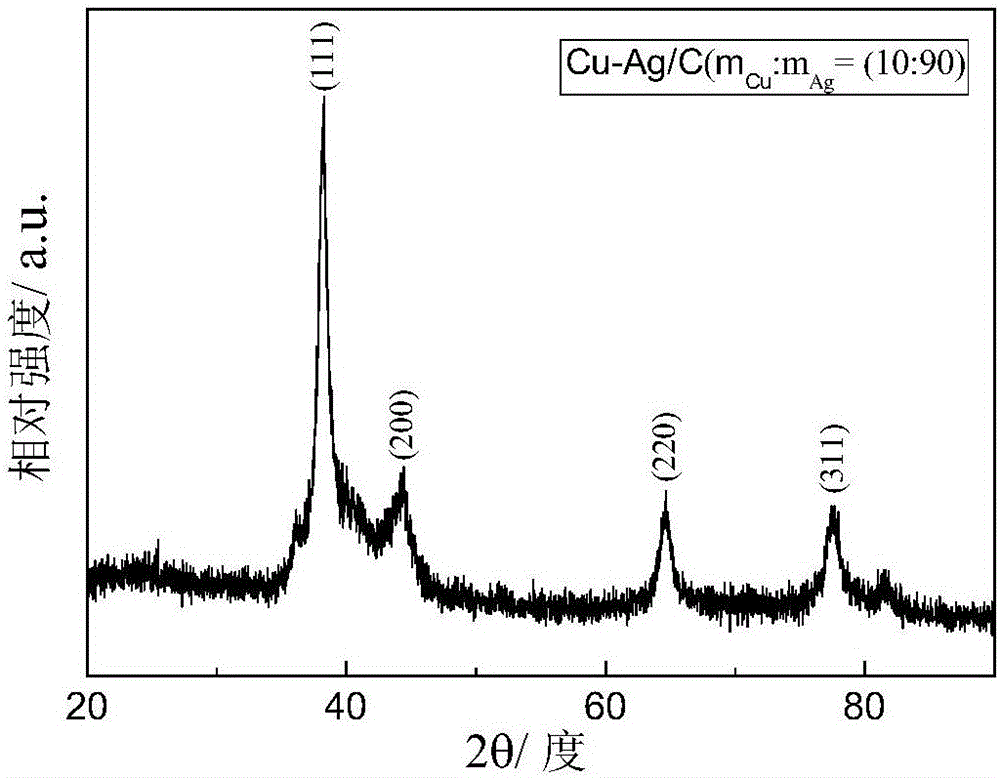
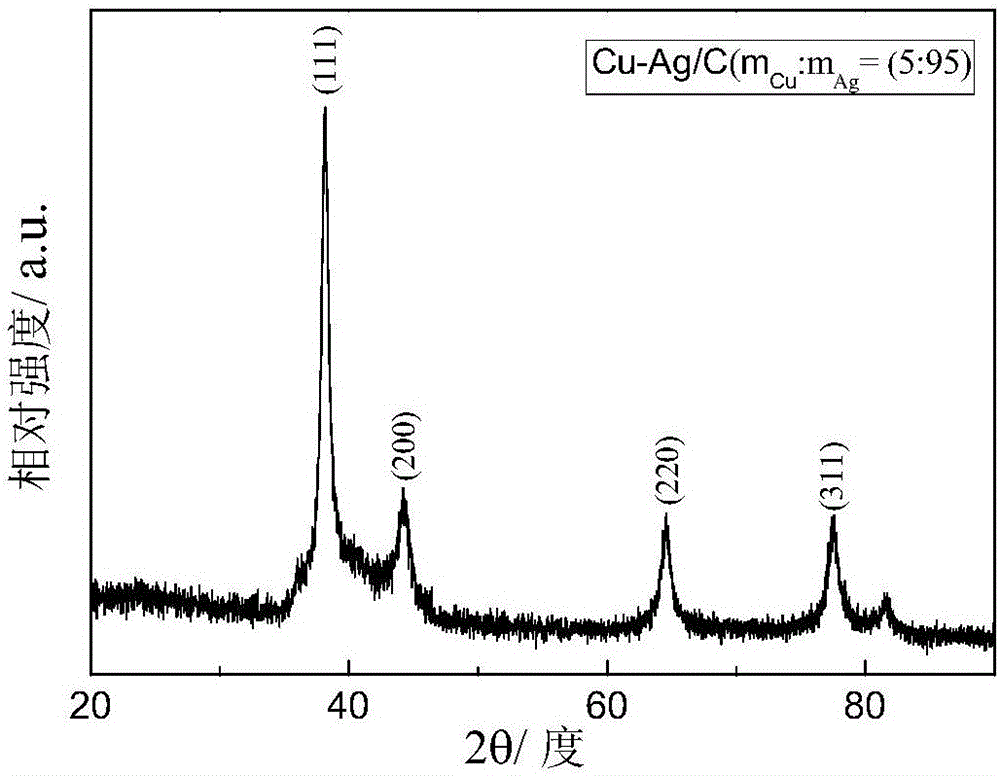
Catalyst for electrochemically reducing carbon dioxide into carbon monoxide and preparation method of catalyst
A technology for carbon dioxide and carbon monoxide, which is applied to the field of copper-silver alloy catalysts for electrochemically reducing carbon dioxide to carbon monoxide and its preparation, can solve problems such as high overpotential, and achieve high CO current efficiency, excellent catalytic activity, and improved CO partial current density. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0037] The catalyst of the present invention is a nano-copper-silver alloy loaded on a carbon carrier, and the preparation method of the catalyst comprises S1: a step of preparing a precursor solution; S2: a step of adding a carbon carrier; S3: a step of wet chemical reduction. However, those skilled in the art can understand that the preparation method of the present invention is not limited to the above-mentioned 3 steps, and in addition to the above-mentioned 3 steps, can also include, for example, the pre-filtering step, cleaning step, drying step and crushing step described later.
[0038] Hereinafter, the above-mentioned steps S1 to S3 and other steps will be sequentially described.
[0039] S1: Prepare the precursor solution step
[0040] Weigh the copper source and silver source compounds according to the mass ratio, and dissolve them in water to form an aqueous solution. The complexing agent is dissolved in water to form an aqueous solution. The aqueous solution of ...
Embodiment 1
[0054] Taking the preparation of Cu-Ag / C in which the copper-silver alloy accounts for 80% of the total mass of the catalyst as an example, copper accounts for 10% of the total mass of the Cu-Ag alloy.
[0055] Weigh 0.171g of silver nitrate and 0.046g of copper nitrate trihydrate and stir to dissolve in 238ml of deionized water. Then 3.485g of sodium citrate dihydrate was weighed, stirred and dissolved in 66ml of deionized water, the two solutions were mixed and magnetically stirred for half an hour, 0.03g of carbon black (VXC-72) was added into it, and ultrasonically dispersed for 1h until uniform dispersion. The above precursor solution was transferred to a Erlenmeyer flask, ice bathed and magnetically stirred, the temperature was controlled at 5°C, and high-purity N 2 Protect. Weigh 0.18 g of sodium borohydride, and configure it into a 5 mmol sodium borohydride solution under ice-bath conditions. Then use a syringe pump to evenly drop it into the precursor solution at a ...
Embodiment 2
[0057] Taking the preparation of Cu-Ag / C in which the copper-silver alloy accounts for 80% of the total mass of the catalyst as an example, copper accounts for 5% of the total mass of the Cu-Ag alloy.
[0058] Weigh 0.171g of silver nitrate and 0.022g of copper nitrate trihydrate and stir to dissolve in 218ml of deionized water. Then weigh 3.209g of sodium citrate dihydrate, stir and dissolve in 60.6ml of deionized water, mix the two solutions with magnetic stirring for half an hour, add 0.029g of carbon black (VXC-72) into it, and sonicate for 1h to uniformly disperse . The above precursor solution was transferred to a Erlenmeyer flask, ice bathed and magnetically stirred, the temperature was controlled at 5°C, and high-purity N 2 Protect. Weigh 0.165 g of sodium borohydride, and configure it into a 5 mmol sodium borohydride solution under ice-bath conditions. Then use a syringe pump to evenly drop it into the precursor solution at a rate of 1ml / min until the addition is c...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Particle size | aaaaa | aaaaa |
| Particle size | aaaaa | aaaaa |
| Size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com