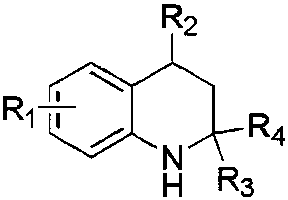
1,2,3,4-tetrahydroquinoline compound and its synthesis method and application
A technology of tetrahydroquinolines and compounds, applied in organic chemistry, drug combination, diseases, etc., can solve the problems of cumbersome steps, and achieve the effects of simple operation, less environmental pollution, and a wide range of substrates
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0039] Synthesis of 2-methyl-7-nitro-1,2,3,4-tetrahydroquinoline-2-carboxylic acid methyl ester:
[0040] Take a 10 mL round bottom flask connected with a reflux condenser, add 2,4-dinitrotoluene (182 mg, 1 mmol), methyl methacrylate (294 mg, 3 mmol) and cesium carbonate (652 mg, 2 mmol), evacuate nitrogen, inject anhydrous THF (5 mL), and heat to 65 o C, reflux for 12 hours. After the reaction, the solvent and excess olefins were removed by rotary evaporation, the remaining solid was washed with sufficient amount of dichloromethane, filtered, and the filter residue was dissolved in sufficient amount of water, then activated carbon was added for decolorization, the solid was filtered off, the solvent was spin-dried, and vacuum-dried to obtain recovered of cesium carbonate. The filtrate containing the product was spin-dried and purified through a silica gel column with dichloromethane as the developing solvent to obtain the target product as a yellow solid with a yield of 213...
Embodiment 2
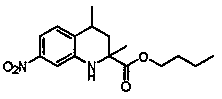
[0046] Synthesis of butyl 2-methyl-7-nitro-1,2,3,4-tetrahydroquinoline-2-carboxylate:
[0047] The reaction raw materials are 2,4-dinitrotoluene (182 mg, 1 mmol), butyl methacrylate (420 mg, 3 mmol), and the reaction time is 12 hours. Other specific operations are the same as in Example 1. The product was a yellow solid, the yield was 237 mg, and the yield was 81%.
[0048] Product infrared spectrum data: IR (KBr): 3382 cm -1 , 2933 cm -1 , 1728 cm -1 , 1616 cm -1 ,1523 cm -1 , 1454 cm -1 , 1346cm -1 , 1180 cm -1 .
[0049] Product H NMR spectrum data: 1 H NMR (400 MHz, CDCl 3 ) δ 7.48 (dd, J = 8.2, 2.1Hz, 1H, HAr), 7.42 (d, J = 2.1 Hz, 1H, HAr), 7.07 (d, J = 8.3 Hz, 1H, HAr), 4.66 (s, 1H, NH), 4.14 (t, J = 6.6 Hz, 2H, CH 2 ), 2.93-2.68 (m, 2H, CH 2 ), 2.34(dt, J = 13.0, 5.2 Hz, 1H, CH 2 ), 1.90 (ddd, J = 13.2, 10.3, 5.9 Hz, 1H, CH 2 ),1.61 (dd, J = 13.3, 5.5 Hz, 2H, CH 2 ), 1.50 (s, 3H, CH 3 ), 1.33 (dd, J = 15.1,7.5 Hz, 2H, CH 3 ), 0.92 (t, ...
Embodiment 3
[0053] Synthesis of 2,4-dimethyl-7-nitro-1,2,3,4-tetrahydroquinoline-2-carboxylic acid methyl ester:
[0054] The reaction raw materials are 2,4-dinitroethylbenzene (194 mg, 1 mmol), methyl methacrylate (294 mg, 3 mmol), and the reaction time is 12 hours. Other specific operations are the same as in Example 1. The product was a yellow solid, the yield was 191 mg, and the yield was 73%.
[0055] Product infrared spectrum data: IR (KBr): 3383 cm -1 , 2932 cm -1 , 1732 cm -1 , 1603 cm -1 ,1535 cm -1 , 1485 cm -1 , 1345 cm -1 , 1181 cm -1 .
[0056] Product H NMR spectrum data: 1 H NMR (400 MHz, CDCl 3 ) δ 7.87 (d, J = 2.3 Hz,1H, HAr), 7.47 (s, 1H, HAr), 7.03 (d, J = 2.2 Hz, 1H, HAr), 4.96 (s, 1H, NH),3.77(s, 3H, CH 3 ), 3.00 (ddd, J = 16.1, 14.6, 9.5 Hz, 1H, CH 2 ), 2.88 (d, J =8.4 Hz, 3H, CH 3 ), 2.81 (d, J = 26.5 Hz, 1H, CH 2 ), 2.32-1.87 (m, 1H, CH 2 ), 1.60(s, 3H, CH 3 ) ppm.
[0057] Product structural formula:
[0058]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com