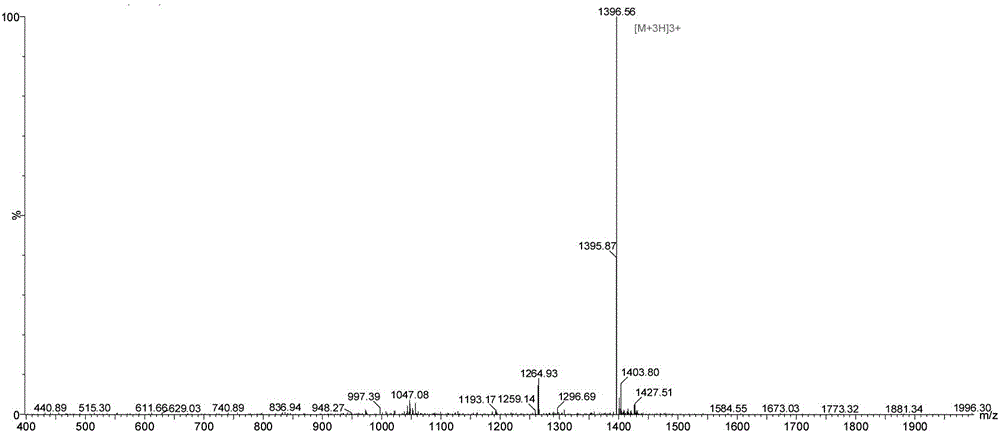
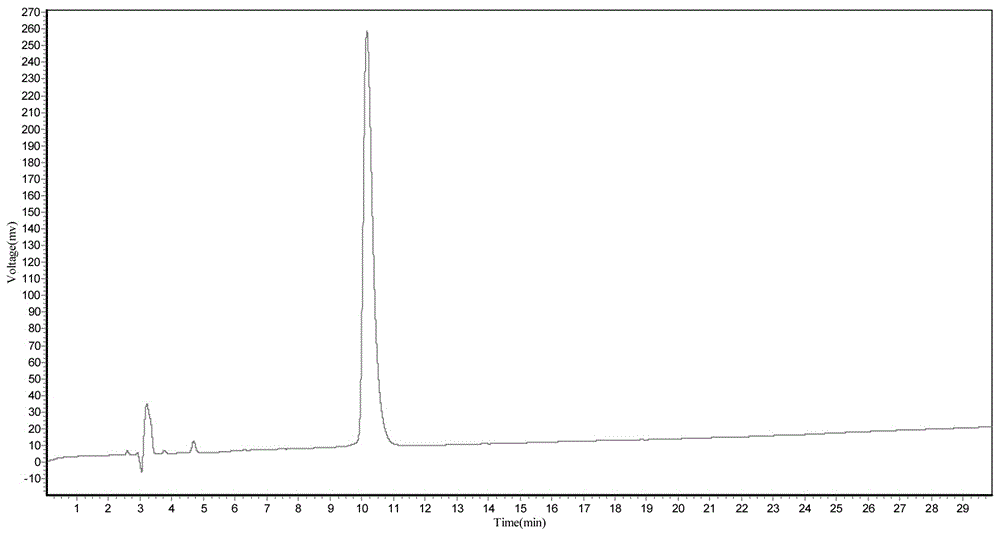
Solid-phase synthesis method for preparing exenatide
A technology for exenatide and solid-phase synthesis, which is applied in the preparation methods of peptides, chemical instruments and methods, peptides, etc., can solve the problems of complex synthesis steps, difficult separation in the later stage, and difficult coupling, etc., and achieves reduction of residual peptides. The formation of impurities, the elimination of β-sheet secondary space structure, and the effect of improving yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0062] (1) Preparation of Fmoc-Rink Amide AM PEGMatrix resin
[0063] Weigh 25 grams of AM PEGMatrix resin (0.8mmol / g, 20mmol), soak it with 500ml DCM for 30 minutes, make the resin fully swell, drain, add Fmoc-Rink Amide linker (MW:539.58, 40mmol) 21.6g, DIEA (MW : 129.24, 80mmol) 13.5ml, TBTU (MW: 321.1, 40mmol) 12.8g, HOBT (MW: 135.1, 40mmol) 5.4g, DMF / NMP 500ml with a volume ratio of 1:1, reacted for 4 hours, drained, and used DMF Wash 3 times, drain, then add 500ml of acetic anhydride:pyridine:DMF mixed solution with a volume ratio of 2:2:1, react for 1 hour, drain, wash 6 times with DMF, and drain to obtain Fmoc-RinkAmide AM PEGMatrix resin.
[0064] (2) Preparation of His(trt)-Gly-Glu(otbu)-{Gly-Thr(psi(me,me)pro)}-Phe-Thr(tbu)-Ser(tbu)-Asp(otbu)-{Leu -Ser(psi(me,me)pro)}-Lys(boc)-Gln(trt)-Met-Glu(otbu)-Glu(otbu)-Glu(otbu)-Ala-Val-Arg(pbf)-Leu -Phe-Ile-Glu(otbu)-Trp(boc)-Leu-Lys(boc)-Asn(trt)-Gly-Gly-Pro-{Ser(tbu)-Ser(psi(me,me)pro)} -Gly-Ala-Pro-Pro-Pro-Pro-Ser(tbu...
Embodiment 2
[0107] Step (1) AM PEGMatrix resin reacted with Fmoc-Rink Amide linker and DIEA for 8 hours, then added acetic anhydride: pyridine: DMF mixed solution to react for 0.5 hours, and the degree of substitution of PEGMatrix resin was 1.0mmol / g; step (2) Fmoc -Rink Amide AM PEGMatrix resin, add deprotection reagent, react for 45 minutes; add Fmoc-Ser(tbu)-OH, NMM, reaction time is 0.5 hour; step (3) Add in the peptide chain resin with fully protected side chain Peptide cutting reagent, react for 2.5 hours. All the other are with embodiment 1.
Embodiment 3
[0109] Step (1) AM PEGMatrix resin reacted with Fmoc-Rink Amide linker and TBTU for 2 hours, then added acetic anhydride:pyridine:DMF mixed solution for 2 hours, the degree of substitution of PEGMatrix resin was 0.5mmol / g; step (2) Fmoc -Rink Amide AM PEGMatrix resin, add deprotection reagent, react for 60 minutes; add Fmoc-Ser(tbu)-OH, NMM, reaction time is 2 hours; step (3) add in the peptide chain resin with fully protected side chain Peptide cutting reagent, react for 3 hours. All the other are with embodiment 1.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com