Trehalose synthase mutant and gene thereof
A technology of trehalose synthase and mutants, applied in genetic engineering, glycosyltransferase, plant gene improvement, etc., can solve the problem of low activity of trehalose synthase
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
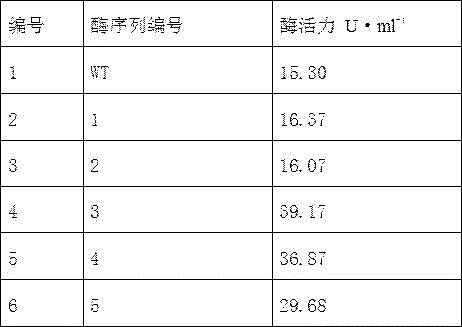
[0018] Example 1 Preparation of Trehalose Synthase Mutants
[0019] according to mycobacterium smegmatis The gene sequence of the source trehalose synthase was designed and synthesized sequentially to introduce five mutants: R169A, R169A-D174A, R169A-D174A-D167A, R169A-D174A-D167A-T175A, R169A-D174A-D167A-T175A-E176A The primers were used to carry out site-directed mutagenesis on the trehalose synthase in five steps, and the DNA coding sequence was determined.
[0020] The Arg codon at position 169 was identified as an Ala codon.
[0021] On the basis of the previous step, change the 174th Asp codon to Ala codon.
[0022] On the basis of the previous step, change the 167th Asp codon to Ala codon.
[0023] On the basis of the previous step, the 175th Thr codon was changed to Ala codon.
[0024] On the basis of the previous step, change the 176th Glu codon to Ala codon.
[0025] The mutant gene was connected with an appropriate expression vector and introduced into Escheri...
Embodiment 2
[0045] Example 2 Expression and Purification of Trehalose Synthase
[0046]The constructed plasmid pET22b-TreS was transformed into E.coliBL21 (DE3) host for expression. A single clone was inoculated in 5 mL of LB liquid medium with an ampicillin concentration of 100 mg / L, and cultured in a shaker at 200 rpm at 37°C for 4 h. Transfer the cultured strains to 1 L of LB liquid medium with an ampicillin concentration of 100 mg / L, culture at 37°C until OD600=0.6, cool down to 30°C and add IPTG (final concentration 0.2 mM) to induce expression for 14 h, 6000 rpm The cells were collected by centrifugation for 10 min and stored in a -80°C refrigerator.
[0047] Take 10 g of frozen bacteria and resuspend in BufferA (25mM Tris, 300Mm Nacl, pH8.0). Use a high-pressure cell disruptor to break the cells, centrifuge at 16,000 rpm for 30 min after breaking, and collect the supernatant; secondly, Ni-NTA affinity chromatography: first use Buffer A to equilibrate the Ni-NTA column, and slowly...
Embodiment 3
[0048] Example 3 Expression of trehalose synthase mutants in Bacillus subtilis
[0049] (1) Construction of Bacillus subtilis expression vector
[0050] Through molecular biological operations such as double enzyme digestion, gel recovery, and T4 ligase connection, five mutants of trehalose synthase were cloned into the pMA5 vector to obtain five different pMA5-TreS, which were prepared for use in Bacillus subtilis. expression.
[0051] (2) Expression of recombinant strain WB600( pMA5-tres)
[0052] Introduce the constructed recombinant vector into the competent Bacillus subtilis B. subtilis WB600, activate the recombinant bacteria WB600, and carry out dilution coating, and then pick a ring of recombinant bacteria with a moderate size of a single colony to further activate in LB, 37°C, 250r / min for overnight incubation. The next day, microscopic examination was performed to see if there was bacterial infection, and then inoculated in LB medium at an inoculation volume of 3...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com