Tetrahexose monosialogandlioside-modified recombinant lipoprotein and application thereof
A ganglioside and monosialic acid technology, applied in the fields of neuropharmacology and chemical pharmacy, can solve the problems of difficult to improve the pathological manifestations of AD patients, neuron immune attack, aggravation, etc., to avoid the leakage of recombinant lipoprotein loaded drugs, The effect of not easy to gather
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
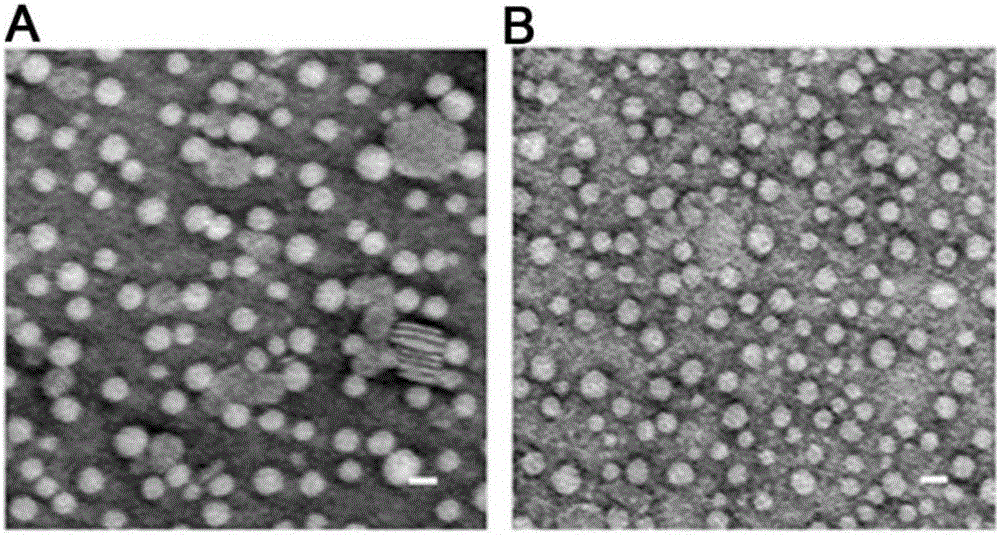
[0045] Example 1. Monosialotetrahexosyl ganglioside modification improves the monodispersity of recombinant lipoproteins
[0046] (1) Preparation
[0047] 5%, 10%, 20% of the total lipid molar percentage of monosialotetrahexosyl ganglioside incorporated into 95%, 90%, 80% of the total lipid molar percentage of dimyristoylphosphatidylcholine In the alkali, add chloroform to dissolve, remove the organic solvent by rotary evaporation under reduced pressure, add pH7.4 phosphate buffer solution hydration to the lipid film, obtain monosialotetrahexosyl ganglioside modified liposome (lipid Total mass 4mg). Add 0.8 mg of ApoE, mix gently, place on a vibrating shaker at 100 rpm and incubate at 37° C. for 36 hours to obtain monosialotetrahexosylganglioside-modified recombinant lipoprotein.
[0048] (2) Characterization
[0049] Laser particle size analyzer measured the particle size and zeta potential. The results showed that the particle size of monosialotetrahexosyl ganglioside mod...
Embodiment 2
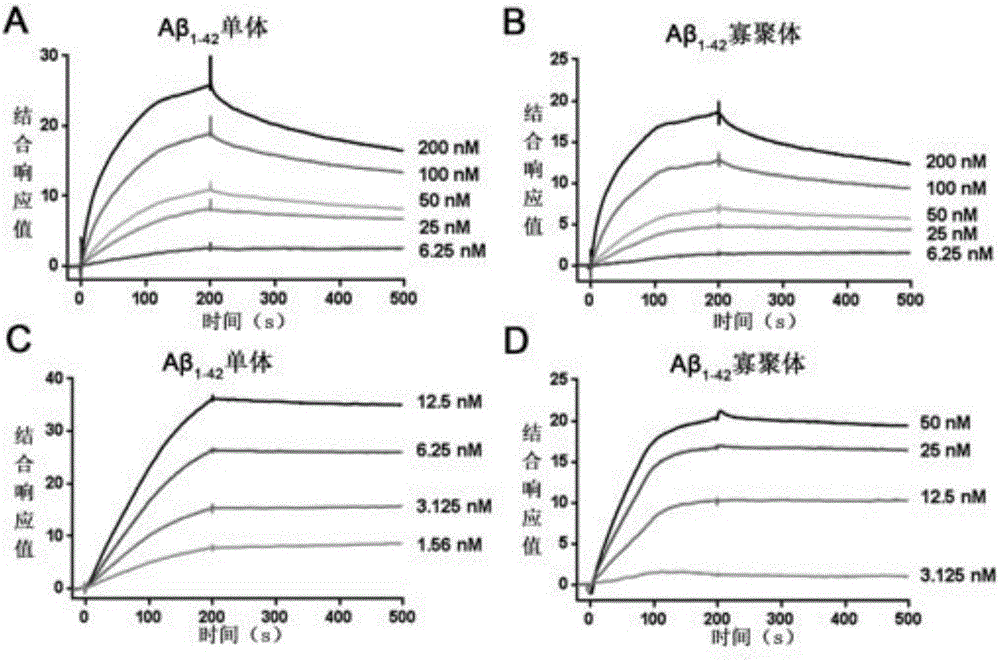
[0051] Example 2. Monosialotetrahexosyl ganglioside modification improves the Aβ affinity of recombinant lipoprotein pairs
[0052] (1) Preparation
[0053] 5%, 10%, 20% of the total lipid molar percentage of monosialotetrahexosylganglioside incorporated into 95%, 90%, 80% of the total lipid molar percentage of dipalmitoylphosphatidylcholine Medium (total lipid mass 4mg), ApoE 0.8mg, same as Example 1 to prepare monosialotetrahexosylganglioside modified recombinant lipoprotein.
[0054] Cardiolipin accounting for 5% of the total lipid molar percentage is incorporated into the dipalmitoylphosphatidylcholine (total lipid mass 4mg) accounting for 95% of the total lipid molar percentage, ApoE 0.8mg, the same as above to prepare cardiolipin modified recombinant lipid protein.
[0055] Accounting for 10% sulfatide in the total lipid molar percentage is incorporated into dipalmitoylphosphatidylcholine accounting for 90% total lipid molar percentage (total lipid mass 4mg), ApoE 0.8 ...
Embodiment 3
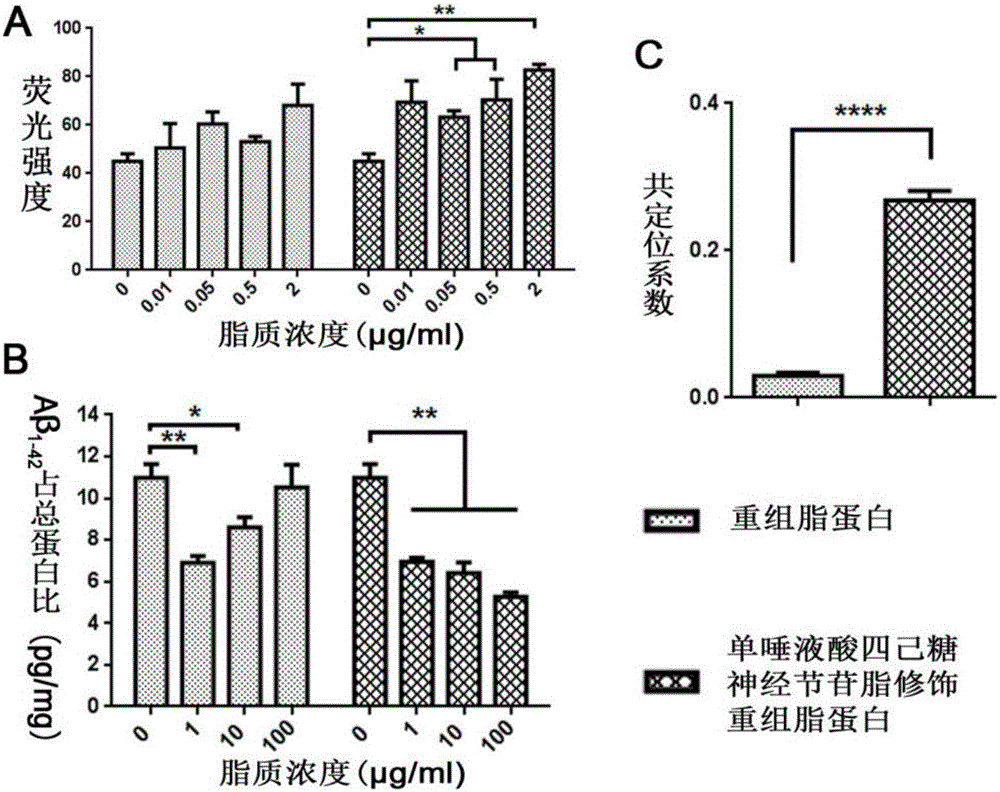
[0061] Example 3. Monosialotetrahexosyl ganglioside modification improves the uptake and degradation of Aβ by recombinant lipoprotein-mediated microglial cells
[0062] (1) Preparation
[0063] The monosialotetrahexosyl ganglioside accounting for 5% of the total lipid molar percentage is incorporated into the phosphatidylcholine and phosphatidic acid mixture (4 mg total lipid mass) accounting for 95% of the total lipid molar percentage, ApoE 0.4 mg, same as in Example 1 to prepare monosialotetrahexosylganglioside modified recombinant lipoprotein.
[0064] (2) Monosialotetrahexosylganglioside modified recombinant lipoprotein promotes the uptake of Aβ by primary microglia
[0065] FAM fluorescently labeled Aβ 1-42 Added to the 96-well plate planted with microglial cells, respectively, the recombinant lipoprotein and monosialotetrahexosylganglioside modified recombinant lipoprotein were diluted with DMEM and added to the 96-well plate planted with primary microglial cells plat...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com