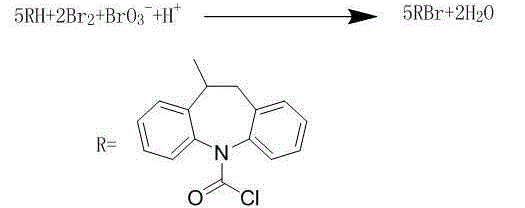
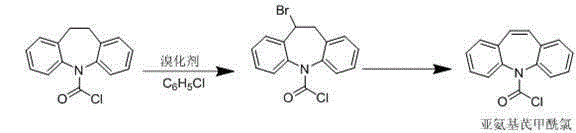
Synthetic method for carbamazepine intermediate iminostilbene carbonyl chloride
A technology of iminostilbenecarbonyl chloride and iminodibenzylcarbonyl chloride is applied in the field of synthesis of pharmaceutical intermediates and can solve the problems of unfavorable labor protection, limited improvement of bromine utilization rate, low bromine utilization rate and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0026] A synthetic method of carbamazepine intermediate iminostilbenoyl chloride, which comprises the steps of: adding chlorobenzene 848ml, iminodibenzoyl chloride 106g (0.410mol), benzoyl peroxide 4.24g and Benzyltriethylammonium chloride 6.36g, the oil bath is heated to 92°C, sodium bromate 13.6 (0.09mol) is added to the reactor, and bromine 28.8g (0.18mol) is added dropwise at a constant speed, and the dropping time is 4.5 h, then raised the temperature to 97°C and refluxed for 4 hours. After the reflux, the reaction solution was cooled to 20°C, poured into 300ml of methanol solution, allowed to stand for 4 hours, filtered, and the filter cake was dried and concentrated under reduced pressure to remove the organic solvent. Dissolve in 848ml of toluene, add activated carbon for recrystallization and decolorization, filter, the filtrate is cooled to crystallize, filter, and vacuum dry to obtain about 95g of yellow solid iminostilbene carboxylic acid chloride, the yield is 90.5...
Embodiment 2
[0028] A synthetic method of carbamazepine intermediate iminostilbenoyl chloride, which comprises the steps of: adding chlorobenzene 848ml, iminodibenzoyl chloride 106g (0.410mol), benzoyl peroxide 4.24g and Benzyltriethylammonium chloride 5.30g, the oil bath is heated to 92°C, sodium bromate 13.6 (0.09mol) is added to the reactor, and bromine 28.8g (0.18mol) is added dropwise at a constant speed, and the dropping time is 4.5 h, then raised the temperature to 97°C and refluxed for 4 hours. After the reflux, the reaction solution was cooled to 20°C, poured into 300ml of methanol solution, allowed to stand for 4 hours, filtered, and the filter cake was dried and concentrated under reduced pressure to remove the organic solvent. Dissolve in 848ml of toluene, add activated carbon for recrystallization and decolorization, filter, the filtrate is cooled to crystallize, filter, and vacuum dry to obtain about 90g of yellow solid iminostilbene carboxylic acid chloride, the yield is 85.7...
Embodiment 3
[0030] A synthetic method of carbamazepine intermediate iminostilbenoyl chloride, which comprises the steps of: adding chlorobenzene 848ml, iminodibenzoyl chloride 106g (0.410mol), benzoyl peroxide 4.24g and Benzyltriethylammonium chloride 8.48g, the oil bath is heated to 92°C, sodium bromate 13.6 (0.09mol) is added to the reactor, and bromine 28.8g (0.18mol) is added dropwise at a constant speed, and the dropping time is 4.5 h, then raised the temperature to 97°C and refluxed for 4 hours. After the reflux, the reaction solution was cooled to 20°C, poured into 300ml of methanol solution, allowed to stand for 4 hours, filtered, and the filter cake was dried and concentrated under reduced pressure to remove the organic solvent. Dissolve in 848ml of toluene, add activated carbon for recrystallization and decolorization, filter, the filtrate is cooled to crystallize, filter, and vacuum dry to obtain about 94g of yellow solid iminostilbene carboxylic acid chloride, the yield is 89.5...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com