Isosorbide-monoimidazole salt compounds and preparation method thereof
A salt compound, isosorbide technology, applied in organic chemistry, drug combination, antipyretic, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
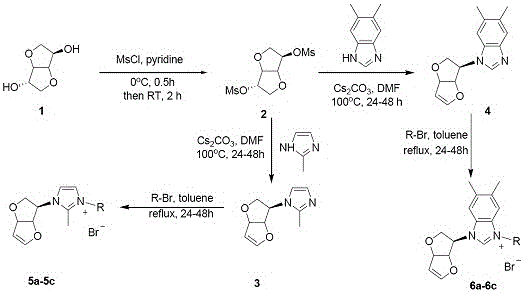
[0018] The preparation method specifically includes:
[0019] A, the preparation of compound isosorbide mesylate:
[0020] Using isosorbide as raw material, mesylate esterification with methanesulfonyl chloride in anhydrous pyridine: dissolve isosorbide in anhydrous pyridine, add methanesulfonyl chloride dropwise at 0 °C, the molar ratio of the dosage is Isosorbide: methanesulfonyl chloride = 1: 2.5, the amount of anhydrous pyridine is 20 ml: 1g isosorbide, after stirring and reacting at room temperature for 20 hours, dichloromethane was added for dilution (30ml: g substrate), and water ( 50 ml) and saturated brine (50 ml), the organic phase was washed with anhydrous NaSO 4 Dry, filter, and concentrate the solvent under reduced pressure, add water (30 ml), raise the temperature to 55°C and stir for 2 hours until the precipitate is completely dissolved, then cool it to room temperature, a white solid precipitates, filter, and use ethanol for precipitation (30 ml) was washed r...
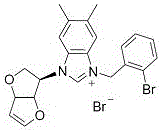
Embodiment 1
[0026] Preparation of compound 5a: see the above preparation methods A, B, and C;
[0027]
[0028] Compound 5a: Formula C 18 h 19 BrN 2 o 3, Yield 90%. White solid powder, mp 181-186 o c. 1 H NMR (300 MHz, DMSO-d 6 ): δ 8.10 (1H, s), 8.08 (1H, s), 7.80-7.73 (2H, m), 7.67-7.62 (2H, m), 7.58 (1H, s), 6.94 (1H, d, J = 2.1 Hz), 6.15 (2H, s), 5.71 (1H,d, J = 4.5 Hz), 5.25-5.18 (3H, m), 4.18 (1H, d, J = 10.8 Hz), 3.72-3.67 (1H, m), 2.69 (3H, s). 13 C NMR (75 MHz, DMSO-d 6 ): Δ 191.04, 150.79, 146.06, 134.51,133.66, 128.96, 128.41, 123.22, 118.70, 99.53, 86.35, 84.21, 62.81,54.92, 54.51, HRMS (ESI-TOF) M / Z Calcd FOR C 18 h 19 N 2 o 3 + [M-Br] + 311.1390, found 311.1389.
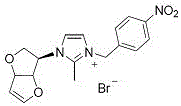
Embodiment 2
[0030] Preparation of compound 5b: see the above preparation methods A, B, and C;
[0031]
[0032] Compound 5b: Formula C 18 h 18 Br 2 N 2 o 3, Yield 82%. White solid powder, mp 164-167 o c. 1 H NMR (300 MHz, DMSO-d 6 ): δ 8.03 (1H, s), 8.00 (1H, s), 7.89 (1H, s), 7.86 (1H, s),7.72 (1H, s), 7.58 (1H, s), 6.93 (1H, d , J = 2.4 Hz), 6.14 (2H, s), 5.70 (1H, d, J = 3.0 Hz), 5.25-5.18 (3H, m), 4.18 (1H, d, J = 10.8 Hz), 3.71-3.66 (1H,m), 2.68 (3H,s). 13 C NMR (75 MHz, DMSO-d 6 ): Δ 190.45, 150.79, 146.08, 132.74,132.03, 130.37, 128.60, 123.18, 118.71, 99.53, 86.33, 84.20, 62.82,54.90, 54.92. HRMS (ESI-TOF) M / Z Calcd FOR COR COR C 18 h 18 BrN 2 o 3+ [M-Br] + 389.0495, found 389.0496.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com