A kind of aescin A injection and preparation method thereof
A technique for aescin and injection, applied in the field of pharmaceutical preparations, can solve problems such as inability to reduce nephrotoxicity, and achieve the effects of good resolubility and quality stability, strong anti-inflammatory, and full appearance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
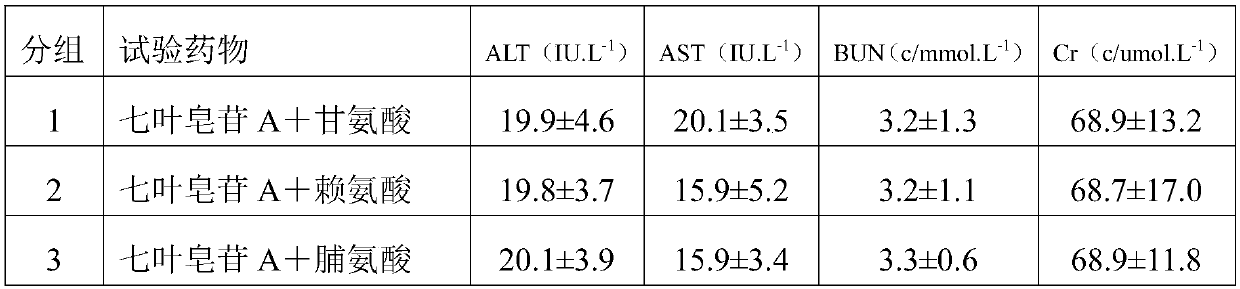
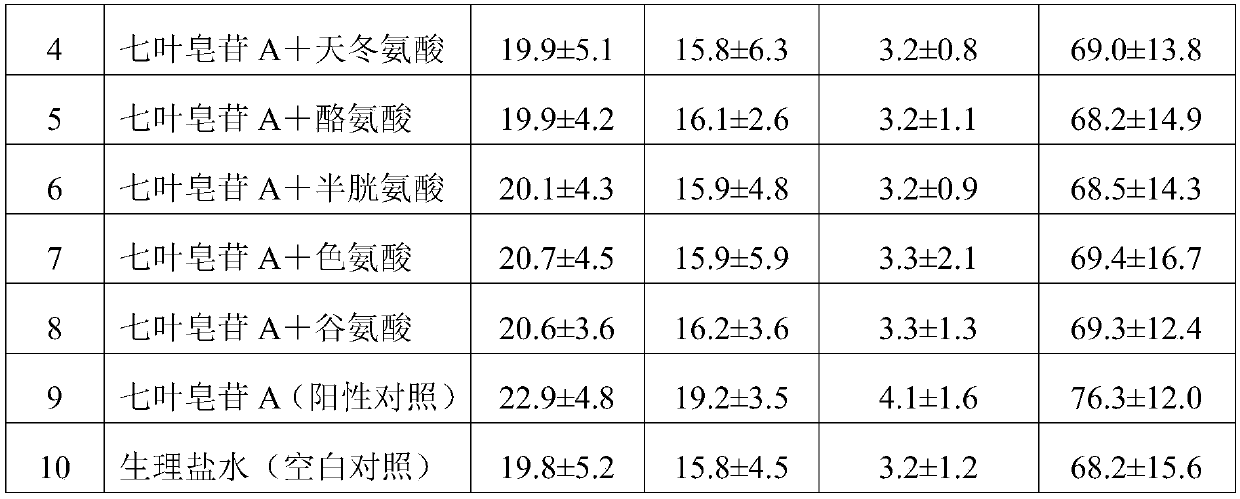
[0029] 1) 30g aescin A, 2g glycine, 200g mannitol are dissolved in 2000ml water for injection to prepare a mixed solution, the concentration of mannitol in the mixed solution is 10g / 100ml, and the concentration of aescin A is 1.5g / 100ml , the concentration of glycine is 0.1g / 100ml;
[0030] 2) adjust the pH value of the mixed solution to 6.5 with lactic acid;
[0031] 3) Filter the solution after adjusting the pH value with a 0.22 μm filter membrane;
[0032] 4) Freeze drying after filling.
[0033] The freeze-drying procedure is as follows:
[0034] Pre-freezing: quickly lower the temperature of the material to -20°C and keep it for 1 hour, then slowly lower the temperature of the material to -40°C and keep it warm for 4 hours;
[0035] Primary sublimation: control the vacuum degree below 20 Pa, raise the temperature of the material to -10°C and keep it warm for 4 hours;
[0036] Re-drying: Raise the temperature of the material to 35°C and keep it warm for 12 hours.
Embodiment 2
[0038] 1) 40g aescin A, 6g lysine, 800g mannitol are dissolved in 4000ml water for injection to make a mixed solution, the concentration of mannitol in the mixed solution is 20g / 100ml, and the concentration of aescin A is 1g / 100ml, the concentration of lysine is 0.15g / 100ml;
[0039] 2) adjust the pH value of the mixed solution to 4.5 with lactic acid;
[0040] 3) Filter the solution after adjusting the pH value with a 0.22 μm filter membrane;
[0041] 4) Freeze drying after filling.
[0042] The freeze-drying procedure is as follows:
[0043] Pre-freezing: first quickly lower the temperature of the material to -25°C and keep it for 0.5 hours, then slowly lower the temperature of the material to -35°C and keep it warm for 5 hours;
[0044] Primary sublimation: control the vacuum degree below 20 Pa, raise the temperature of the material to -5°C and keep it warm for 3 hours;
[0045] Re-drying: Raise the material temperature to 40°C and keep it warm for 10 hours.
Embodiment 3
[0047] 1) 25g aescin A, 5g aspartic acid, 150g mannitol are dissolved in 1250ml water for injection to prepare a mixed solution, the concentration of mannitol in the mixed solution is 12g / 100ml, and the concentration of aescin A is 2g / 100ml, the concentration of aspartic acid is 0.4g / 100ml;
[0048] 2) adjust the pH value of the mixed solution to 5.5 with lactic acid;
[0049] 3) Filter the solution after adjusting the pH value with a 0.22 μm filter membrane;
[0050] 4) Freeze drying after filling.
[0051] The freeze-drying procedure is as follows:
[0052] Pre-freezing: first rapidly lower the temperature of the material to -22°C and keep it for 0.5 hours, then slowly lower the temperature of the material to -38°C and keep it warm for 3 hours;
[0053] Primary sublimation: control the vacuum degree below 20 Pa, raise the temperature of the material to -15°C and keep it warm for 5 hours;
[0054] Re-drying: Raise the material temperature to 30°C and keep it warm for 15 ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| concentration | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com