Method for preparing graphite phase carbon nitride nanosheet through airtight oxidation
A technology of graphite phase carbon nitride and nanosheets, which is applied in chemical instruments and methods, nitrogen compounds, nanotechnology, etc., can solve the problems of less product structure defects, high yield, and fewer steps, and achieve good morphology and high yield. The effect of high efficiency and low cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0032]Add 0.6g of graphite phase carbon nitride, 3g of potassium permanganate, and 30ml of concentrated sulfuric acid to the polytetrafluoroethylene lining in sequence. Tighten the stainless steel reaction kettle, seal it, and place it in the refrigerator (0-5°C) for 1.5h. After taking it out, tighten the reactor again and place it in an oven (80°C) for heating for 1.5h. Then take out the reactor and cool to normal temperature. Dilute with 400ml deionized water, and add 30% hydrogen peroxide while stirring until the solution becomes pure white and stop adding. Finally, it was washed with 600ml of 5% hydrochloric acid and 1000ml of deionized water to neutrality, and dried in an oven (60°C).
[0033] figure 1 The laser image of the graphitic phase carbon nitride nanosheet prepared for the present invention, from figure 1 It can be seen that the product obtained in the experiment will produce Tyndall effect when irradiated with laser light, indicating that the size of the pro...
Embodiment 2
[0035] Add 0.6g of graphite phase carbon nitride, 3g of potassium permanganate, and 30ml of concentrated sulfuric acid to the polytetrafluoroethylene lining in sequence. Tighten the stainless steel reaction kettle, seal it, and place it in the refrigerator (0-5°C) for 1.5h. After taking it out, tighten the reactor again and place it in an oven (80°C) for heating for 1.5h. Then take out the reactor and cool to normal temperature. Dilute with 400ml deionized water, and add 30% hydrogen peroxide while stirring until the solution becomes pure white and stop adding. Filter and wash with deionized water until neutral, and dry in an oven (60°C). 100mg of graphitic carbon nitride nanosheets were obtained.
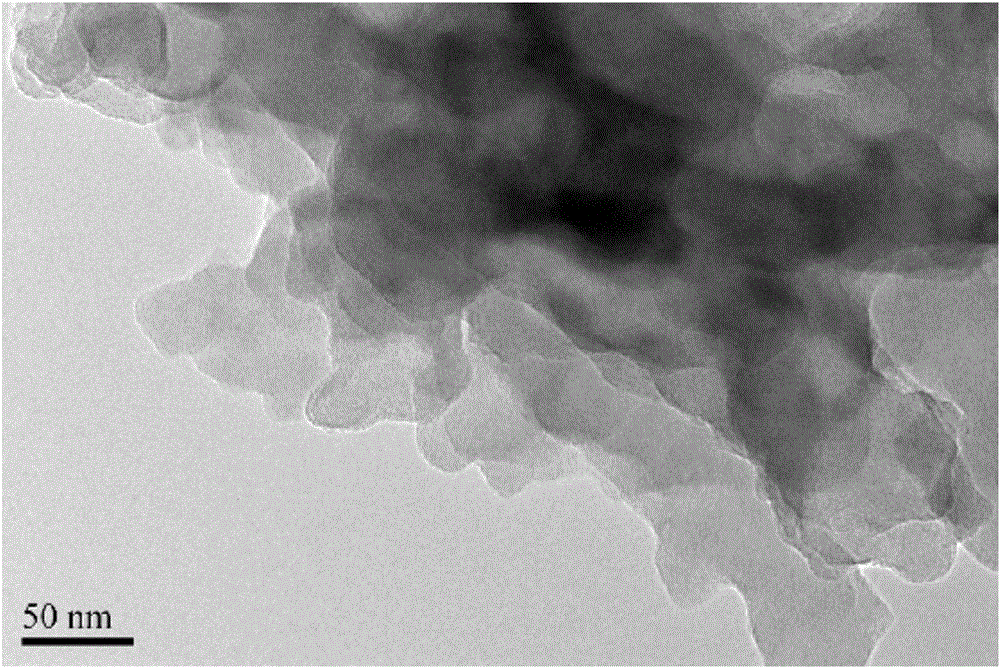
[0036] figure 2 Transmission electron microscopy (TEM) of graphitic phase carbon nitride nanosheets prepared for the present invention.
Embodiment 3
[0038] Add 0.6g of graphite phase carbon nitride, 3g of potassium permanganate, and 30ml of concentrated sulfuric acid to the polytetrafluoroethylene lining in sequence. Tighten the stainless steel reaction kettle, seal it, and place it in the refrigerator (0-5°C) for 1.5h. After taking it out, tighten the reactor again and place it in an oven (80°C) for heating for 1.5h. Then take out the reactor and cool to normal temperature. Dilute with 400ml deionized water, and add 30% hydrogen peroxide while stirring until the solution becomes pure white and stop adding. Finally, it was washed with 600ml of 5% hydrochloric acid and 1000ml of deionized water until neutral, and freeze-dried.
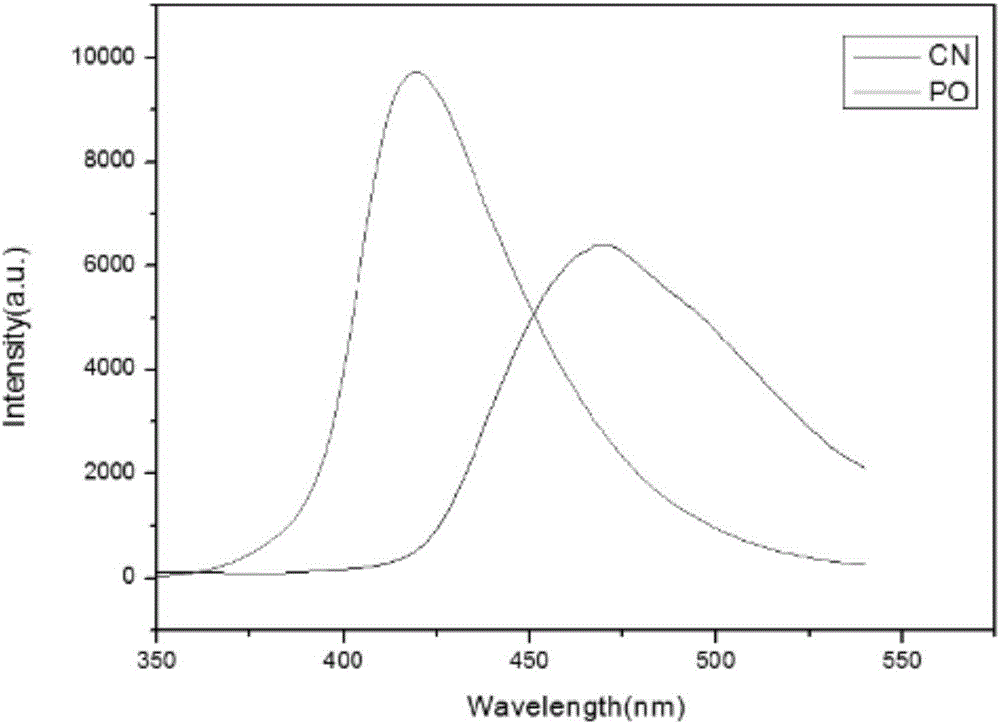
[0039] image 3 Fluorescence figure (FL) of the graphitic phase carbon nitride nanosheet prepared for the present invention, from image 3 It can be seen that the fluorescence intensity of graphitic carbon nitride is significantly stronger than that of graphitic carbon nitride.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com