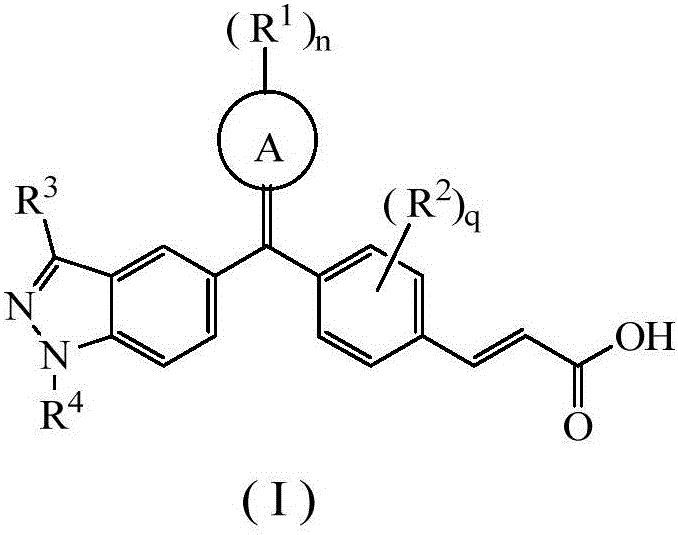
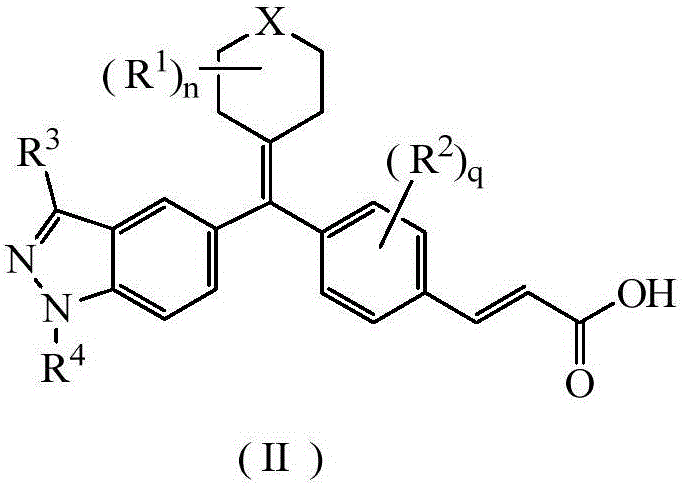
Acrylic acid derivative, preparation method therefore and medical application of acrylic acid derivative
A technology of compounds and mixtures, applied in the field of medicine, can solve problems such as hot flashes, venous thrombosis, and endometrial hyperplasia
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
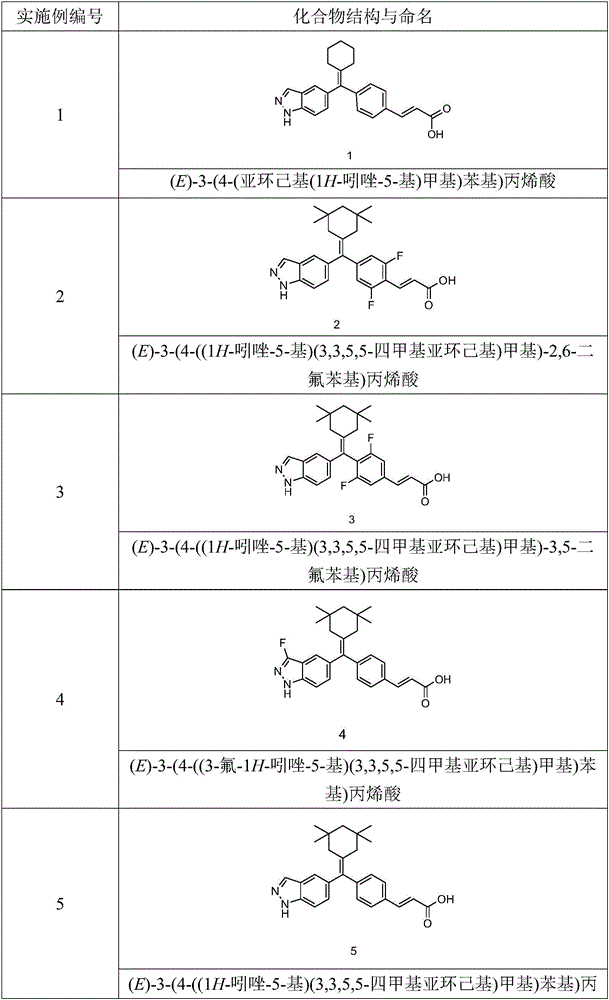
Embodiment 1
[0144] (E)-3-(4-(cyclohexylene(1H-indazol-5-yl)methyl)phenyl)acrylic acid
[0145]
[0146] first step
[0147] (4-bromophenyl)(1-(tetrahydro-2H-pyran-2-yl)-1H-indazol-5-yl)methanol
[0148] 5-Bromo-1-(tetrahydro-2H-pyran-2-yl)-1H-indazole 1a (500mg, 1.8mmol, using the known method "Journal of Organic Chemistry, 2009, 74(16), 6331 -6334" prepared) was dissolved in 20mL tetrahydrofuran, cooled to -78°C, n-butyllithium (0.8mL, 1.98mmol) was added dropwise, stirred for 5 minutes, and pre-made 3mL p-bromobenzaldehyde (300mg, 1.62 mmol) in tetrahydrofuran, stirred and reacted at -78°C for 2 hours. After the reaction, add 10mL saturated ammonium chloride solution to quench the reaction, extract with ethyl acetate (50mL×2), dry the organic phase with anhydrous sodium sulfate, filter, concentrate the filtrate under reduced pressure, and use silica gel column chromatography to elute Purification of the resulting residue with Reagent System B afforded the title product (4-bromophe...
Embodiment 2
[0172] (E)-3-(4-((1H-indazol-5-yl)(3,3,5,5-tetramethylcyclohexylene)methyl)-2,6-difluorophenyl)acrylic acid
[0173]
[0174]
[0175] first step
[0176] (4-(Benzyloxy)-3,5-difluorophenyl)(1-(tetrahydro-2H-pyran-2-yl)-1H-indazol-5-yl)methanol
[0177] 5-Bromo-1-(tetrahydro-2H-pyran-2-yl)-1H-indazole 1a (1.87g, 6.65mmol) was dissolved in 30mL tetrahydrofuran, and 2.5M n-butyl was added dropwise at -78°C Lithium base (3.0mL, 7.32mmol), stirring for 10 minutes after the dropwise addition, added dropwise 5mL of pre-made 4-(benzyloxy)-3,5-difluorobenzaldehyde 2a (1.65g, 6.65mmol, using the patent application " WO2014069963" prepared by the method disclosed) tetrahydrofuran solution, naturally raised to 25 ° C, stirred and reacted for 12 hours. After the reaction was completed, 20 mL of saturated ammonium chloride solution was added to quench the reaction, extracted with ethyl acetate (20 mL×3), the organic phase was concentrated under reduced pressure, and the resulting re...
Embodiment 3
[0202] (E)-3-(4-((1H-indazol-5-yl)(3,3,5,5-tetramethylcyclohexylene)methyl)-3,5-difluorophenyl)acrylic acid
[0203]
[0204] first step
[0205] (4-bromo-2,6-difluorophenyl)(1-(tetrahydro-2H-pyran-2-yl)-1H-indazol-5-yl)methanol
[0206] Dissolve 5-bromo-1-(tetrahydro-2H-pyran-2-yl)-1H-indazole 1a (560mg, 2mmol) in 10mL ether, add 2.5M n-butyllithium dropwise at -78°C (1.7mL, 2.2mmol), stirred for 10 minutes after the addition was completed, added dropwise 2mL of a pre-made ether solution of 4-bromo-2,6-difluorobenzaldehyde (442mg, 2mmol), naturally rose to 25°C, and stirred for 60 Hour. After the reaction was completed, 10 mL of saturated ammonium chloride solution was added to quench the reaction, extracted with ethyl acetate (10 mL×3), the organic phase was concentrated under reduced pressure, and the resulting residue was purified by silica gel column chromatography with eluent system B to obtain the title product (4-Bromo-2,6-difluorophenyl)(1-(tetrahydro-2H-pyran-2...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com